Fig. 16.1
Factors influencing adequate surgical margins and acceptable cosmetic results in BCS. Without regard to free and safe margins, expertise of operating surgeon and observance of oncoplastic techniques are considered equally critical in order to achieve better results
Operating surgeon, in some circumstances, may represent a prognostic factor, as believed in the past and now scientifically and statistically accepted.
Acceptable cosmetic outcome is directly related to potential residual disease and inversely related to extension of adequate surgical margins.
Oncoplastic techniques, for some selected groups of patients (e.g. too large or small breast), could strikingly improve the overall results.
8.
One en bloc specimen. The surgeon should aim to perform excision in one complete specimen and mark the specimen for the pathologist. Margin assessment by so-called touch prep-imprint cytology or random shave biopsies from the cavity might be helpful, but has not proven to be superior over a complete careful assessment of the wide local excision specimen.
9.
Staging of axilla. In the case of proven axillary lymph node disease, axillary lymph node clearance should be undertaken; if there is no proven disease, the optimal axillary procedure is a sentinel lymph node biopsy, or, if not available, axillary node sampling is an alternative.
10.
Multidisciplinary group. Breast surgeons who act alone are an anomaly that should be eliminated. The best treatment is the one that comes out after a discussion among several people about several options has been carried out.
The surgeon is a member of the MDT and should participate in regular multidisciplinary reviews for case management and audit purposes. MDT is now involved mainly in primary therapy, and it is hoped that, in future, MDT will more involved also in the diagnostic phase. Preoperative MDT discussion is not mandatory but preferable:
To select optimal treatment based on guidelines and clinical criteria
To select patients for non-standard treatment based on individual patient needs and tumour-related factors (e.g. older patients with low-risk BC)
To document proposed treatments (medicolegal issues)
To select patients for clinical trials
To treat rare histological cases, associated diseases and their individual characteristics
NON–STANDARD TREATMENTS may be required for the following three groups and should be identified and properly well thought out.
Unifocal operable tumours too large for BCS for which downsizing with neoadjuvant systemic therapy could be considered (see Sect. 18.4). However, systemic therapy for histologically confirmed invasive BC too large for BCT should be carefully monitored. In fact, a reasonable possibility is that neoadjuvant therapy may efficiently eliminate highly differentiated tumour cells and less so poorly differentiated ones that may be in a dormant state and reactivate under specific circumstances.
Inflammatory BC, where neoadjuvant chemotherapy is the standard of care (see Sect. 15.2). In other locally advanced BC (e.g. with massive nodal involvement), results are still debatable except for some molecular BC subtypes like triple-negative, HER2-positive and some luminal B subsets (Sect. 18.4).
BRCA carriers. In healthy BRCA mutation carriers, risk- reducing surgery, such as bilateral mastectomy and oophorectomy, needs to be discussed as part of the initial assessment (see Sect. 2.3).
In this last particular case of BRCA mutation carriers with diagnosed BC the oncological safety of BCT is still debated. Based on current evidence, BCT can be considered a reasonable option since it does not seem to increase the risk for IBTR in BRCA-mutation carriers versus non-carriers. However, several aspects should be taken into account before the final decision-making, including therapeutic and cosmetic outcomes of bilateral mastectomy.
The same applies to the high risk patients with a strong family history of BC. What is more, in very young patients with BC, rapid BRCA testing is indicated if knowledge of the mutation status would impact the BC treatment plan. Nevertheless, if the patient needs more time to decide on any possible prophylactic surgery or if a rapid test is not available, stage-adapted treatment of BC should be performed while postponing any potential prophylactic procedure to a second stage.
Lastly, outside of the genetic or familiar risk, a woman with a personal history of BC has a 3 to 4-fold increased risk of developing a new cancer in the other breast or in another part of the same breast. However, that is not a sufficient reason for implementing prophylactic measures as contralateral surgery, unless in certain special situations as pluricentric lobular carcinoma or atypical hyperplasia.
16.1.2 Quality Indicators in Breast Surgery
Almost all guidelines highlight operative measures aimed at obtaining best oncological results without neglecting some minor aspects of quality assurance [1, 2].
Unnecessary surgery. To minimise unnecessary surgery, i.e. open surgical diagnostic biopsies that prove to be malignant, invasive BCs should have a nonoperative pathological diagnosis in at least 90 % of cases. For non-invasive BC, the minimum standard for a nonoperative pathological diagnosis is 85 %.
Cosmesis. To minimise the cosmetic impairment of diagnostic open biopsy, the fresh weight of tissue removed for all cases where a diagnostic open biopsy is performed should be recorded. It is recommended that more than 90 % of open surgical biopsies carried out for diagnosis, which prove to be benign, should weigh less than 20 g.
Anxiety. To minimise patient anxiety regarding a diagnostic operation which is required to confirm or exclude malignancy and the date for an operation, patients should be admitted for a diagnostic operation within 2 weeks in more than 90 % of cases.
Delay. To minimise the delay between referral for investigation and first BC treatment, 100 % of patients diagnosed with BC should receive their first treatment within 62 days of an urgent referral with suspected BC or recall from the screening programme. This is valid whether surgery is the first treatment or neoadjuvant systemic therapy is planned.
Timing. To minimise patient anxiety regarding a therapeutic operation, which is required for cancer and the date of the actual operation, 100 % of patients should receive their first treatment within 31 days of the decision to treat. This is valid whether surgery is the first treatment or neoadjuvant systemic therapy is planned.
Preoperative investigations. Finally, to minimise unnecessary investigations prior to BC treatment, nonoperative staging investigations for metastatic disease should not be routinely performed.
Minimal number of operations. The proportion of patients (invasive cancer only) who receive a single (breast) operation for the primary tumour (excluding reconstruction) should be a minimum of 70 %.
16.2 Breast-Conserving Surgery
Clinical Practice Points
The goals of BCT are to provide a survival equivalent to mastectomy, a cosmetically acceptable breast and a low rate of recurrence in the treated breast.
Breast-conserving therapy (BCT) is a combination of breast-conserving surgery (BCS) aimed at microscopically free margins and radiotherapy (RT) of the breast.
The patient should be fully informed that breast irradiation is required following conservation and that further surgery may be required if the margins are not clear of tumour.
In BCS the radial tumour margins must be clear (>1 mm) in order to keep the breast relapse rate of invasive cancer lower than 1–1.5 % per follow-up annum (less than 10 % at 10 years).
While a slight asymmetry may be tolerated, a deformity is to be avoided. Wide margins do not appear to achieve better local control rates and may impair cosmetic results.
Patients who have had BCS and have a poor cosmetic outcome can be offered partial breast reconstruction to improve results.
16.2.1 Introduction
For patients with single small BCs, survival outcomes from breast-conserving treatment (BCT) are equivalent to that of mastectomy. That is true only in the absence of local recurrence which should be avoided. Although in the past it was thought that local therapy had little influence on overall survival, it is becoming clear that local failure is responsible, at least in part, for some patients developing metastatic disease [3].
Completeness of excision is the major surgical factor influencing local recurrence. The size of the lesion and the size of the excision are related to the size of the breast. No upper size limit for breast-conserving surgery (BCS) for invasive cancer can be given.
The appropriate selection of patients is crucial to the success of BCS, which it is not applicable to all patients. Mastectomy is mandatory for tumour control for some subgroups of patients with BC, and it may provide more satisfactory outcomes in others.
Contraindications for BCS, and consequently absolute indication for mastectomy, are:
Multicentric disease with two or more primary tumours in separate quadrants of the breast such that they cannot be encompassed in a single excision.
Diffuse malignant microcalcifications on mammography.
A history of prior therapeutic RT that would result in an excessively high total radiation dose to the chest wall.
Extensive skin changes, a clinical diagnosis of inflammatory BC and dermal lymphatic involvement.
In pregnancy, however, it may be possible to perform breast-conserving surgery in the third trimester, deferring breast irradiation until after delivery.
Persistently positive resection margins after multiple attempts at re-excision.
Individual patient’s preference. The appropriate selection starts from individual patient needs and expectations that should be accurately assessed. There are many issues to consider regarding this decision: benefits and risks of mastectomy compared to BCS in regard to specific concerns, possibility and consequence of local recurrence with either treatment, impact on cosmetic outcome and psychosocial adjustment.
16.2.2 Selection Criteria for BCT
Preoperative assessment. Imaging typically includes a combination of bilateral mammographic evaluation, with appropriate magnification views if needed, and ultrasound. The size of the tumour should be included in the mammographic report, as well as documentation of associated microcalcifications, and the extent of the calcifications within and outside the mass. Some surgeons incorporate breast MRI in the workup of patients considering BCT; however, the use of MRI in this setting is not routinely indicated.
MRI for the preoperative assessment of disease in newly diagnosed BC is indicated:
For a clinical presentation of disease that is larger than what is appreciated by mammography, particularly in the setting of dense breasts which lower the sensitivity of mammography
For invasive cancers that are contiguous to the chest wall and not completely included on mammographic projections
For patients with axillary nodal metastases and a clinically and mammographically occult primary tumour
For women with Paget disease of the breast who have a negative physical examination and mammogram
In women with locally advanced BC who are being considered for neoadjuvant systemic therapy to assess tumour response to therapy
For women with very high risk for contralateral disease because of an inherited predisposing condition or prior chest wall irradiation
Women should be informed of the risks and benefits of preoperative breast MRI (see Sect. 5.3). The limits of the accuracy of MRI should be discussed with patients, so that they understand the need for biopsy of MRI-detected lesions before definitive surgery. Breast MRI should be performed with a dedicated breast coil by expert breast imaging radiologists in institutions that have the capability to perform MRI-guided needle biopsy and/or wire localisation of the findings.
Surgical decisions should not be based on MRI findings alone. All suspicious findings on MRI require pathologic confirmation. MRI findings alone should not be used to change surgical plans and to shift from breast conservation to mastectomy.
Age is not a contraindication to BCT in very young as well as in older women. Younger patients (<40 years of age) have an increased risk of local recurrence after BCS as well as after a mastectomy, but there is no evidence to suggest that wider than no ink surgical margins on the tumour reduces the local recurrence risk in this population (see Sect. 15.1). For older women, the primary determinants of local therapy should be physiologic age and presence of comorbid conditions (see Sect. 15.4).
Family history of BC is not a contraindication to BCT; however, women with a strong family history suggestive of a genetic predisposition should be informed about their increased risk of a second primary cancer.
Dense breast tissue is not, in itself, a contraindication for BCS. If patients with dense breasts are more likely to be treated with an initial mastectomy, this may reflect the surgeon’s or the patient’s bias rather than an inability to meet the criteria for BCS. In fact, breast density is not associated with higher positive margins, and preoperative MRI does not decrease the risk of positive margins [4].
Tumour location should not influence the choice of treatment. Tumours in a superficial subareolar location may require resection of the nipple-areolar complex to achieve negative margins. In that case only cosmetic, no oncologic, outcomes will be affected. If anything, it is important to establish that the tumour is unicentric and clearly localised.
Tumour size – Tumour size in itself is not an absolute contraindication to BCS. A large tumour in a small breast is a relative contraindication, since an adequate resection would result in significant cosmetic alteration. In patients with unifocal operable tumours too large for BCS, downsizing with neoadjuvant systemic therapy should be considered in order to reduce tumour size and allow for breast conservation with acceptable rates of local recurrence. Metallic clip placement in the tumour bed under ultrasound guidance should be accomplished before or soon after neoadjuvant treatment begins in order to properly identify the location in case there is a complete response to chemotherapy.
There is no role for preoperative chemotherapy in patients with invasive BC who are already candidates for BCS or have dispersed microcalcifications and multifocal diseases. At this moment neither the optimal combination nor duration of chemotherapy has been clearly evaluated; thus, preoperative chemotherapy to downsize the tumour in order to facilitate BCT should be applied with caution.
Lymph node metastases are a marker of worse prognosis, but positive lymph nodes are not a contraindication for BCS, as BCT and mastectomy have equivalent outcomes independent of nodal metastases. In general, patients with aggressive biological factors do not benefit from wider margins.
Retraction of the skin, nipple or breast parenchyma is not necessarily a sign of locally advanced BC and does not contraindicate BCS. Only cosmetic implications of a larger resection should be factored into the decision to proceed with BCS.
Histologic subtypes other than invasive ductal carcinoma (e.g. invasive lobular cancer) are not associated with an increased risk of BC recurrence; these women are candidates for BCT if the tumour distribution is not dispersed and it can be excised with negative margins.
Extensive intraductal component (EIC), if present, is an indicator that disease extent may be greater than clinically suspected. EIC is not a contraindication for BCS, providing negative margins are achieved. There is no evidence to support a wider margin than no ink on tumour when all suspicious microcalcifications identified on the mammogram in the vicinity of the cancer have been resected. If residual microcalcifications are present, a wider excision should be attempted.
Connective tissue disease – Some patients with a history of connective tissue disease tolerate irradiation poorly, and so the use of RT as a component of BCT must be weighed against the possible complications. However, large studies noted that patients with scleroderma and systemic lupus erythematosus (SLE), but not rheumatoid arthritis, are at a significantly increased risk of late toxicities. As a result, many radiation oncologists consider only scleroderma and active SLE to be relative contraindications to BCT.
16.2.3 Techniques of BCS
Wire localisation – For resection of non-palpable lesions, preoperative wire localisation by the radiologist allows accurate identification of the area requiring resection (see Sect. 6.4.2). Multiple bracketing wires may be helpful for the delineation of the boundaries of the resection, but do not ensure clear histologic margins of resection.
Incision – The type and location of the incision can be critical to the quality of cosmesis for several reasons. First, any patient who undergoes lumpectomy may ultimately require a mastectomy, and incisions should be planned with possible mastectomy incisions in mind. Moreover, the incision should be placed close to the tumour to avoid extensive tunnelling that could impair tumour bed boost radiotherapy.
In the upper part of the breast, incisions should be curvilinear or transverse and follow the natural skin creases (Langer’s lines). Radial incisions in the upper half of the breast may displace the ipsilateral nipple-areola complex due to scar contracture. In the lower part of the breast, the choice of a curvilinear or radial incision is dependent upon the contour of the breast, the distance from the skin to the tumour and the amount of breast tissue to be resected. Generally, skin removal with a curvilinear incision in the inferior part of the breast distorts the breast contour and may displace the nipple-areolar complex downward. At 3 and 9 o’clock position and in the lower breast, a radial or curvilinear incision provides a better result, particularly if skin removal is necessary (Fig. 16.2).

Fig. 16.2
In the upper part of the breast, curvilinear skin incisions following Langer’s lines generally achieve the best cosmetic result. Only in the lower part of the breast, a radial or oblique incision may provide a good result. Particularly in the lower pole locations, the effective mobilisation of the gland, as shown here, is a key in achieving a natural breast shape
The incision should be over the tumour and of adequate size to allow the tumour to be removed in one piece. In the upper inner aspect of the breast, some retraction of the skin may be necessary to avoid an incision that may be visible with clothing.
In deeper lesions, it is not necessary to remove the skin. Preservation of the subcutaneous fat and avoidance of thin skin flaps are important in maintaining a normal posttreatment breast contour. If the tumour is superficial, it may be necessary to remove the overlying skin and, if it is deep, the underlying fascia. For both conditions, resection should provide a reasonable distance of 5–10 mm. Finally, needle track from CNB or FNS does not need to be removed.
Glandular resection – Cosmetic outcomes can be improved with oncoplastic techniques; however re-approximation of the breast tissue without tissue advancement is best avoided since it can result in distortion of the breast contour, which may not be apparent with the patient supine on the operating table.
Facing small non-palpable BC without a definite preoperative diagnosis of cancer, the surgeon may be tempted to perform biopsies wider than necessary in the hope that the surgery can be both diagnostic and curative. This is risky if it is negative and is associated with an undesirable aesthetic outcome, and if positive, the excision turns out to be inadequate. In both cases, the doors are open to litigation. All this confirms the necessity to have a definitive preoperative diagnosis.
Margins of resection – The optimal amount of normal tissue that should surround the tumour to minimise the risk of a local recurrence is still controversial. The appropriate macroscopic margin of normal breast tissue to resect around the tumour for women should be 0.5–1.0 cm of grossly normal breast tissue, which will usually result in histologically negative margins (>1 mm distance between tumour and ink) in the majority of patients. There is no evidence that a wider margin of normal tissue than no ink on the tumour decreases the rate of local recurrence in the clinical setting of multimodality treatment. Factors independently associated with positive resection margins included mammographic microcalcifications, tumour size, presence of EIC, grade 2/3 and caudal location of the lesion.
Obtaining tumour-free resection margins is more challenging for non-palpable breast cancer. Models to predict margins status in patients undergoing BCS for non-palpable invasive breast cancer have been developed, which are moderately able to differentiate between women with high versus low risk of positive margins, and may be useful for surgical planning and preoperative patient counselling [5].
Several approaches can be used for intraoperative margin assessment, including frozen section, cytological touch prep analysis, shaved margin analysis and intraoperative ultrasound. There is no prevailing standard of care for intraoperative margin assessment, and practices vary widely. The use of these techniques may assist in obtaining negative margins, but does not guarantee the absence of microscopic tumours on permanent sections. Patients should be advised that additional surgery to obtain clean margins may be necessary.
Specimen orientation – The specimen should be removed as a single piece of tissue. The lesion should be well centred with equidistant margins of resection (Fig. 16.3). Sometimes gross inspection of the specimen in the operating room, with or without frozen section analysis, permits identification of positive or close margins and immediate re-excision, if appropriate [6]. This will decrease the need to return to the operating room for re-excision. The operating surgeon to orient the specimen uses sutures, clips and/or multicoloured inks.
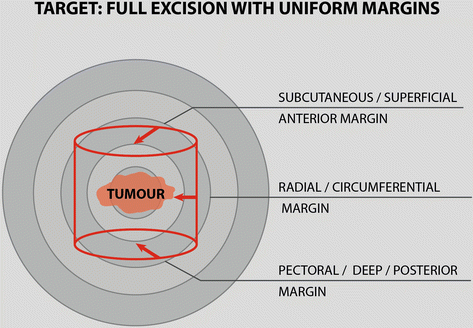

Fig. 16.3
Tumour in the specimen should be well centred with uniform margin of resection
An alternative to removing the entire tumour and margins as a single specimen is to remove the lesion intact and then to resect (to shave) individual margins of normal-appearing tissue that completely encompasses the primary cancer site [7]. Each margin is labelled and oriented in relationship to the primary cancer.
Specimen radiography – Specimen radiography should be performed during the surgical procedure to confirm excision of the targeted lesion when the lesion was not palpable. Additional oriented margins can be resected prior to closure when radiography suggests inadequate resection, which may eliminate the need for a delayed re-excision.
16.2.4 Outcomes of BCS
Pathological results – The first aim of BCS is to obtain radial clear tumour margins (>1 mm) in order to keep the breast relapse rate of invasive cancer lower than 1–1.5 % per follow-up annum (<10 % at 10 years). If pathological results prove a higher risk for breast relapse (incompletely excised infiltrating or in situ cancer, impossibility to deliver an adequate dose of radiation therapy), a re-excision (when cosmetically feasible) or mastectomy must be considered. To minimise the number of therapeutic operations in women undergoing conservation surgery for an invasive cancer, all patients (or at least 95 %) should have less than three operations.
Cosmetic outcome – Another aim of BCS is to result in an acceptable cosmetic appearance in the majority of women. There is a direct correlation between cosmetic outcome after BCS and psychological morbidity. Better cosmetic outcomes reduce the levels of psychological morbidity, with less anxiety and depression and improved body image, sexuality and self-esteem, compared with mastectomy [8]. An all-inclusive good result should be achieved considering both oncological procedures and aesthetic details (Table 16.1).
Table 16.1
Oncological procedures and aesthetic (but important) details to allow a safe BCS and a satisfactory cosmetic outcome
Oncological procedures | Aesthetic details |
|---|---|
Increasing tumour size does not associate with increasing local recurrence rates. So that limiting BCS to cancer below a certain size is illogical | Incision that follows the lines of maximum resting skin tension produces the most cosmetically acceptable scar |
Patients having BCS are adequate treated by wide local excision and do not require either a segmental or quadrantic excision | Routine excision of skin when performing an excision cannot be justified in most cases |
Factors independently associated with positive resection margins include mammographic microcalcifications, tumour size, presence of DCIS, grade 3 and caudal location of the lesion | Effective mobilisation of the gland is a key component in achieving a natural breast shape. Drains and approximation sutures should be avoided or kept to a minimum |
Full-thickness excision ensures free anterior and posterior margins, leaving only the lateral margins in question | Skin staples and interrupted suture do not produce satisfactory results and are not an acceptable method of wound closure in the breast |
Excellent or good cosmetic result from a patient’s point of view should be at least 80 % at 3 years. Many surgical factors will play a role in the ultimate cosmetic appearance of the breast. These include the size and placement of the incision, management of the lumpectomy cavity and the extent of axillary dissection if necessary. The surgeon has control over several of these issues, and careful attention to detail will improve the aesthetic results. Although treatment-related changes in the breast stabilise at approximately 3 years, other factors that affect the untreated breast, such as change in size because of weight gain or the normal ptosis seen with aging, continue to affect breast symmetry.
To help assess the cosmetic outcome after BCT, a scoring system to standardise the grading of cosmetic outcome has been recommended in order to define results as: excellent, the treated and untreated breast are almost identical; good, minimal differences between the treated and untreated breasts; fair, obvious differences between the treated and untreated breasts; and poor, major aesthetic sequelae in the treated breast.
While a slight asymmetry may be tolerated, a deformity is to be avoided. Wide margins do not appear to achieve better local control rates and may impair cosmetic results [9]. Avoiding deformity should be the objective parameter of quality of cosmesis and breast deformities recorded in the chart as follow:
Grade I – Patient has a treated breast with a normal appearance, but there is asymmetry between the two breasts.
Grade II – Patient has a deformity of the treated breast. This deformity can be corrected by partial breast reconstruction and breast conservation, with the irradiated breast tissue being spared in the reconstruction.
Grade III – Patient has a major distortion of the treated breast or diffuse painful fibrosis. These sequelae are so severe that only a mastectomy can be considered.
The most important factor influencing cosmetic outcome after BCS is the percentage of volume of breast excised. Removing more than 20 % of breast volume results in the majority of women having a poor cosmetic outcome. In a trial from the National Cancer Institute of Milan that compared quadrantectomy to gross tumour excision (lumpectomy), women undergoing quadrantectomy had significantly greater discrepancies in the inferior profile of the breasts and greater distance from the midline to the nipple and were more likely to have more than a 3 cm difference in height between the nipples (21 % versus 7 %) than those treated with lumpectomy [10].
Similar data were noted in other series, as in one large study by the Breast Health Center of Boston that correlated cosmetic outcome to the volume of breast tissue resected (estimated by multiplying the dimensions of the resected breast specimens). For women with <35 cm3 resected tissue, excellent and excellent or good scores were reported by 85 and 96 %, respectively. In a later series, cosmetic results declined over the first 3 years and then stabilised; they were still judged by clinicians to be excellent in 77 %, good in 9, fair in 9 and poor in 5 % of cases [11].
16.2.5 Nonsurgical Options for a Breast-Conserving Treatment
Accessibility and diffusion of BC screening, together with improvement of new technologies, have led to the detection of smaller and earlier-stage BCs, where lumpectomy still remains the absolute standard of care for the local treatment. However, given a trend towards less aggressive treatment of small BCs and the ability to target non-palpable lesions, the development of less invasive alternatives seems a more logical alternative than surgery with promising effectiveness and less morbidity [12]. Therefore, the next inevitable step in the evolution of BC therapy could be the replacement of lumpectomy with nonsurgical methods for destroying the tumour, at least for a select group of patients, as those older than 70 years or with comorbidities that make surgery a difficult and unpleasant treatment.
Minimally invasive ablation techniques have been studied in early-stage small tumours with the goal of attaining efficacy similar to that of breast conservation therapy. These techniques have several potential advantages, including lower cost of care and simplifying treatment, increased patient comfort and superior cosmetic results with less scarring, better preservation of breast tissue and faster recovery time.
There are five types of thermal ablations that have been or currently are in research clinical trials: cryoablation, radiofrequency, laser, microwave and high-intensity focused ultrasound (HI FU) ablation. The first 4 methods destroy cancers using percutaneous image-guided probe placement.
With HI FU, the operator, rather than creating an incision to remove the tumour, uses magnetic resonance imaging (MRI) or ultrasound guidance to identify the tumour and to direct a focused beam of acoustic energy through the skin into the tumour. This beam heats and destroys the tumour without damaging nearby structures or tissues. A follow-up MRI can determine whether the entire tumour has been ablated/destroyed, and if necessary, focused ultrasound can be repeated [13].
However, as for other thermal ablations, because there is no surgical removal of tissue involved, there are some potential drawbacks to focused ultrasound treatment:
It does not allow for laboratory verification of complete removal of the tumour.
It does not produce extensive samples to enable analysis of the tumour, so that adjuvant therapy will be planned only on basis of previous NCB.
16.3 Mastectomy
Clinical Practice Points
Mastectomy is indicated for patients who are not candidates for BCT, patients who prefer mastectomy and for prophylactic purposes to reduce the risk of BC.
Some indications to mastectomy are still uncertain, but extensive microcalcifications on the preoperative mammogram are a risk factor for local recurrence after conservation surgery.
As for BCS, the aim of mastectomy is to achieve tumour-free margins. This result may be difficult to obtain in presence of skin or muscle involvement, vascular invasion and extensive modal involvement.
Reconstructive surgery should be available in all cases of mastectomy, and the technique of mastectomy should be appropriate to the method of reconstruction.
16.3.1 Introduction
A mastectomy is the en bloc removal of all breast parenchyma, usually including parts of overlying skin with the nipple-areola complex. Several indications for mastectomy are considered.
Patient not eligible for BCS – Mastectomy remains a reasonable option to achieve local control in invasive BC for patients who are not eligible for BCT (see Sect. 16.2) and in case of patient’s preference. The patient should be informed about this option, including the possibility of immediate breast reconstruction. Breast reconstruction can be offered, but may not delay or hamper locoregional treatment.
Patient’s choice – Some patients may choose to have a mastectomy rather than BCS for various reasons, including a desire to avoid the need for postoperative radiation, further screening or biopsies, as well as to reduce the risk of local recurrence. Patients should be presented with the advantages and disadvantages of the two approaches when both BCS and mastectomy are clinically and oncologically acceptable. This should include discussion of cosmetic concerns, because BCS may result in unacceptable cosmetic results if the patient has a small amount of breast tissue.
Risk–reducing measure – For patients with hereditary breast and ovarian syndromes and patients with mutations of the BC type 1 and 2 susceptibility genes (BRCA1 and BRCA2), a prophylactic mastectomy reduces the risk of developing BC by more than 90 %. Skin-sparing mastectomy with or without preservation of the nipple-areolar complex and immediate reconstruction provides superior cosmetic results for these patients without oncologic compromise. A contralateral mastectomy may be indicated for patients who have been diagnosed with unilateral BC and carry a deleterious BRCA1 or BRCA2 mutation. Otherwise, there is little survival benefit for a prophylactic contralateral mastectomy (see Sect. 2.3).
Locally advanced BC – In extensive disease (either clinically or after histological workup of the excisional specimen), mastectomy may not result in sufficient local control. Factors associated with a high risk for local recurrence after mastectomy are:
Invasive tumour >5 cm (measured by pathologist)
Vascular invasion
Skin or muscle involvement
Involved or close (<1 mm) surgical margins
Extensive nodal involvement (4 or more positive nodes)
Management of the axilla – The management of the axilla is performed following the same workup of all other procedures. For patients with a clinically positive ALN (including ultrasound identified suspicious nodes), the standard of care is a complete axillary dissection, performed at the time of mastectomy.
For patients undergoing a SLN dissection (i.e. clinically negative disease) with node-negative disease or micrometastatic disease (≤2 mm disease), no further axillary dissection is necessary. For patients with a positive SLN, the management of the axilla is evolving, although most surgeons typically would perform a complete ALND at the time of the mastectomy, while other oncologists prefer to plan administration of radiation to the chest wall and axilla. However, trends are changing based upon data extrapolated from management of the axilla in patients treated with BCS.
16.3.2 Techniques for Mastectomy
Radical mastectomy. A radical mastectomy (Halsted mastectomy) consists of en bloc removal of the breast, the overlying skin, the pectoralis major and minor muscles and the entire axillary contents (level I, II and III nodes). The curative potential of this operation remains limited, and the attempts to further expand the field of resection by including the internal mammary nodes failed to improve survival. Radical mastectomy is rarely used unless the tumour has invaded the pectoral muscle.
Modified radical mastectomy. A modified radical mastectomy (MRM) consists of the complete removal of the breast and the underlying fascia of the pectoralis major muscle along with the removal of the level I and II axillary lymph nodes. Survival rates are equivalent to radical mastectomy but with less morbidity.
Simple (or total) mastectomy. A simple mastectomy is the removal of the entire breast, with preservation of the pectoral muscles and the axillary contents. With the emergence of sentinel node biopsy, simple mastectomy is performed more frequently than a modified radical mastectomy.
Skin–sparing mastectomy




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








