Indications
Notes
After breast-conserving surgery
Should be performed in almost all subsets
No subsets are identified which can safely avoid RT
Benefit is minimal in older women with T1 N0 RE+ BC
After mastectomy for large or deep tumours
All T4
All T3 if resection margins are positive or with more risk factors
All T with tumour involvement at deep margin (fascia or muscle)
After mastectomy in the presence of lymph nodes involvement
Recommended in patients with four or more positive axillary lymph nodes at a high risk of local recurrence
Considered for patients with one to three lymph nodes involved at an intermediate risk of local recurrence
After mastectomy in the presence of DCIS
Patients with DCIS identified at the surgically excised margins on final pathologic analysis as the sole indication for postmastectomy RT
After mastectomy following neoadjuvant chemotherapy
The role of RT has not been conclusively demonstrated but is suggested in any pre-chemotherapy stage III and in the presence of residual disease in the breast or in the nodes
After mastectomy triple-negative BC, also in the absence of nodal involvement
The role of RT has not been conclusively demonstrated, but its benefits are suggested from the presence of more negative prognostic factors
17.1.1 Patients Treated with Breast-Conserving Surgery
Most patients treated with BCS are candidates for breast RT. Efforts to identify groups for whom radiation is not indicated because their prognosis is highly favourable or for whom RT is not effective have not been successful. One exception to this includes older women (≥70 years) with node-negative, stage I BC who are treated with endocrine therapy. For these women, the risk of an in-breast recurrence is quite low for them to derive much of a benefit from RT.
For almost all women treated with BCS external beam, whole-breast radiation therapy (WBRT) is indicated. Especially for women deemed to be at a relatively higher risk of local recurrence, a radiation therapy (RT) boost is added to the tumour bed to further reduce the risk of an in-breast tumour recurrence [1].
Whole-breast radiation therapy (WBRT). The benefit of WBRT is supported by large meta-analysis studies, which result that WBRT gives:
A significant reduction in the 10-year risk of recurrence by nearly half of BCS patients, if compared with BCS alone
A significant reduction in the 15-year risk of BC death
These data suggest that about one death from BC is avoided for every four recurrences avoided. Moreover, the benefits of RT were seen in women regardless of whether or not there was evidence of pathologically involved regional nodes [2].
In addition to the survival advantages seen with RT, meta-analysis also reported that first recurrence patterns differ depending on whether or not RT is administered. For women treated with surgery alone, the majority of first recurrences were loco-regional and not distant (25 versus 10 %). In contrast, for women treated with adjuvant RT after surgery, the first recurrence is more commonly distant because the disease is well controlled locally.
Conventionally dosed WBRT is delivered to the entire field (including the whole breast and regional nodes as indicated) in 1.8–2 Gy daily fractions over 4–5 weeks to a total dose of 45–50 Gy.
A shorter fractionation scheme, which reduces the duration of treatment, may be reasonable for properly selected patients. In general, a shorter fractionation scheme delivers 39–41.6 Gy in approximately 3 weeks with or without a boost. In 2011 the ASTRO panellists [3] stated that whole-breast hypofractionated radiotherapy is appropriated for selected women, such as those:
Aged 50 years or older
With pT1-2 N0 BC
With oestrogen-receptor (ER)-positive disease treated primarily with adjuvant hormonal therapy
Compared with conventional regimen, shorter fractionation resulted in:
No difference in the risk of an ipsilateral local recurrence at 10 years – no significant difference in overall survival
A significant decrease in acute and/or late radiation toxicity
Other studies confirm shorter fractionation schedules are safe and effective, and there is no difference in the loco-regional relapse rate (considering distant relapses as well as disease-free survival and overall survival) with shorter fractionation scheme 39–41.6 Gy. At 10 years, treatment to 39 Gy resulted in a significantly less rate of breast induration compared with treatment at 41.6 or 50 Gy, while it resulted in a lower incidence of telangiectasia and breast oedema [4].
The data from these two trials were compiled in a meta-analysis which showed there was no significant difference between the shorter fractionation and conventionally dosed RT schedules, and this was irrespective of age, type of primary surgery, axillary node status, tumour grade, administration of adjuvant chemotherapy or the use of a tumour-bed boost RT. Despite these results, several caveats apply:
Additional studies are needed to more fully evaluate both efficacy and toxicity in particular subgroups, particularly in women with large breasts and/or more advanced tumours as those larger than 5 cm or with positive lymph nodes.
There are insufficient data to evaluate the tolerability of shorter fractionation with other therapies (e.g. chemotherapy or monoclonal antibodies) [5].
Radiation therapy boost to the tumour bed. While RT to the tumour bed following BCS and WBRT is recommended in younger women, its routine use in older women is less clear. RT boost following WBRT is strongly indicated especially if patients have high-risk factors for recurrence as age <50 years old, pathologically involved axillary nodes, lymphovascular invasion and/or close or positive resection margins [6].
Whether to include a tumour-bed boost for patients who undergo shorter fractionation WBRT is controversial and is left to the discretion of the treating physician.
If an RT boost is administered, 10–16 Gy in either 2 Gy or 2.5 Gy fractions is usually administered. Two trials that evaluated the impact of an RT boost suggest that it results in a lower rate of recurrences and, as a result, a lower rate of subsequent mastectomies; however, there is no appreciable benefit in overall survival. Other data support the role of an RT boost and underscore the importance of discussing the role of an additional dose with younger women treated with WBRT, especially if they are deemed to be at high risk of a local recurrence.
Accelerated partial-breast irradiation (APBI). Accelerated partial-breast irradiation refers to the use of limited, focused RT as an alternative to conventional WBRT for women with early BC following BCS. Compared with WBRT, APBI delivers a higher dose of RT per day to a limited volume of tissue over a shorter period of time. Although APBI is a promising alternative to WBRT, there are still limited data to support the role of APBI as an alternative to conventional WBRT. Several sets of published guidelines are mainly focused on which patients should be considered reasonable candidates for treatment with APBI [7, 8]. Partial-breast irradiation may be considered an acceptable treatment option in patients older than 50 years with unicentric, unifocal, node-negative BC up to 3 cm in size and with negative margins of at least 1 mm. Lobular-type BC and the presence of an extensive intraductal component or lymphovascular invasion are still on debate.
Different techniques of APBI are external beam radiation therapy, conformal external beam radiotherapy, intensity-modulated radiotherapy, brachytherapy and intraoperative radiation therapy. The last two techniques are areas of particular attention [9].
Brachytherapy . Brachytherapy for BC involves the temporary placement of radioactive material into body tissues for local radiation treatment. Brachytherapy can be delivered with interstitial or intracavitary delivery systems (Fig. 17.1).
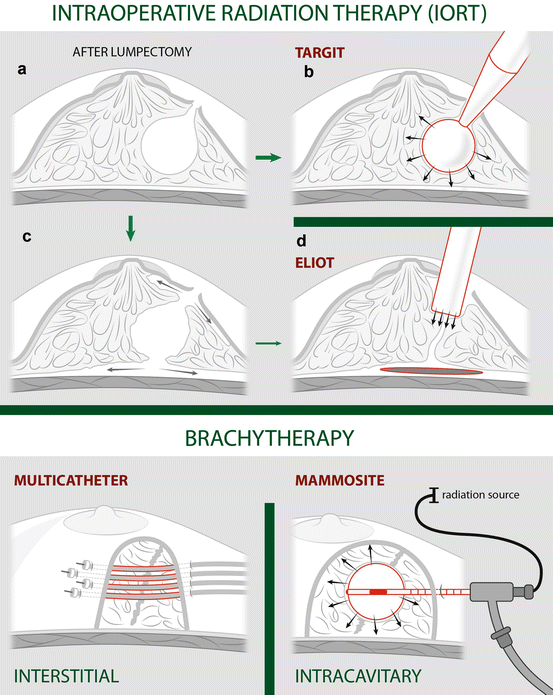

Fig. 17.1
Thumbnails of different types of accelerated partial-breast irradiation (APBI). On the top, intraoperative radiation therapy (IORT) following lumpectomy (a): with low-energy photons (50 kV) from a miniaturized x-ray generator (INTRABEAM) (b), with electrons from a dedicated linear accelerator after a dual-plane glandular undermining (c) on closely spaced margins (d), with a lead shield placed on the pectoralis fascia to protect the lung. On the bottom, brachytherapy, interstitial via multicatheter (e) or intracavitary, as with MammoSite balloon (f)
For interstitial brachytherapy (Fig. 17.1a), several small hollow catheters are placed into the breast surrounding the lumpectomy site. Potential disadvantages of this approach include the risk of infection and poor cosmesis with scarring due to the multiple catheters, although these have been seen in older studies.
For intracavitary brachytherapy (Fig. 17.1b), a radiation delivery device is placed into the partial mastectomy site. Single-lumen and multi-lumen balloon catheter and non-balloon devices have all been used successfully. The presumed advantage of the multi-lumen devices as compared with single-lumen catheters is more precise dosimetric planning and safer treatment delivery, avoiding skin and other organ damage. The device can be placed at the time of lumpectomy (open technique) or several days later under ultrasound guidance (closed technique). The closed technique is preferable because, if the device is placed during surgery, final pathology results may require the device to be removed due to involved margins or positive lymph nodes.
Intraoperative radiation therapy (IORT). Ongoing research is aimed at exploring other modalities of RT that will minimize toxicity without reducing effectiveness. An example of this is intraoperative radiation therapy (IORT), which condenses the entire therapeutic dose into a single fraction, permitting surgery and radiation to be completed in 1 day. At this time, however, IORT should not be administered out of a clinical trial because the available data suggest that IORT is associated with a higher risk of in-breast tumour recurrences (IBTR). For patients in whom the duration of therapy required for standard WBRT is a concern, shorter fractionation schedules are a viable option.
Several devices have been used for intraoperative radiation. A portable radiation-generating device is placed into the lumpectomy cavity (Fig. 17.1c) just after specimen resection, and purse-string sutures are placed within the breast to ensure that the breast tissue is in contact with the applicator surface. Treatment is then delivered with low-energy x-rays, for a dose of approximately 20 Gy at the applicator surface and 5 Gy at a depth of 1 cm over 20–45 min, depending upon the size of the cavity and the device.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree