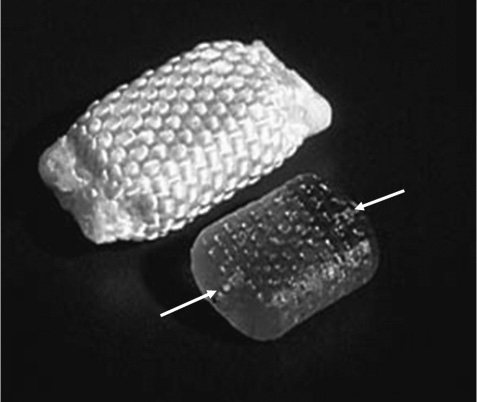
14 The Raymedica prosthetic disk nucleus (PDN; Raymedica, Inc., Minneapolis, MN) has undergone substantial polymeric, mechanical, biocompatibility, toxicological, and clinical implantability research and development (R&D) for several years. These and subsequent product modifications have required nearly 16 years of R&D to define and determine the validity of the author’s vision regarding this specific device and its applicability for reasonably and painlessly restoring and reproducing the natural functions lost in painful degeneration of lumbar intervertebral disks.1 In achieving these goals the need for more complicated obliterative fusion or total disk replacement procedures in selected patients is eliminated. An estimated 4000 patients have been implanted internationally to date using the PDN device, either in its earlier paired design or in the more recent singular PDN-SOLO device.2,3 International commercial use of any medical device results in difficult and sometimes impossible collection of clinical data, or the data often arrive slowly and not always completely. Therefore, definitive information with follow-up examinations has been obtained in only several (~12 of 20) of the earliest cases going back as far as over 9 years and ~300 of the more recent cases. These data generally were obtained following standard protocols with some local variation. But a more recent, controlled cohort is under way using standardized clinical outcomes assessment methods (listed later in this chapter) approved by the U.S. Food and Drug Administration (FDA). The PDN implant is neither a strictly mechanical device nor a free intradiskal polymer or metallic construct or total disk replacement. It is a true nuclear prosthesis that performs much as the original, natural central intradiskal nucleus tissue. Thus this long-term implantable device is unique.4,5 A few more recent nucleus replacement devices have been reported at clinical congresses, but no substantial clinical data have yet been published. In general, for restoring normal or nearly normal conditions of body structures it is preferable to replicate the natural structure and function and not simply to obliterate them. Through contemporary biological and materials sciences, solutions to regeneration or replacement of tissues and structures or organs arise from technologies outside of, but parallel to, medicine and surgery. Technical details of the PDN implants are reviewed following here. Utilizing a protocol with well-recognized outcomes criteria, adults in good general health yet substantially disabled by single-level diskogenic low back pain (although a few two-level cases were performed), with or without herniated disks, and often with substantial accompanying leg pain, were selected. Basically, they were candidates for consideration of an inter-body fusion or a diskectomy, being not quite so symptomatically advanced in back pain as to require a fusion but more advanced than for a simple diskectomy. The primary function of the PDN implant in replacing the diseased, surgically removed central nucleus is to swell through postimplantation hygroscopic expansion, lifting the disk space, tightening the annulus fibers, and restoring essentially normal function.6 It was known that annular stretch through intradiskal lifting pressure keeps the fibers tight, providing ~80% of the normal stability of the segment. The initial design of the PDN device required a dual prosthesis implant because the perforation of the annulus was kept small, and yet the footprint requirement of the final implant replacing the original diskal nucleus was wider than that which a single implant at the time could provide. Thus two smaller devices were implanted side by side and then tethered together inside the nucleus cavity to prevent dislodgment. Nonetheless, the dislodgment rate was initially ~24% and clearly unacceptable. After two additional shape changes and more recently formulation and fabrication changes, the present, singular implant, the PDN-SOLO, was developed. The singular PDN-SOLO implant, made of a modification of the original, remarkably biostable copolymer formulation, described later, was molded and dehydrated using a new production method. When inside the disk cavity, the device rehydrates and swells to the initial size through its polymeric memory, resulting in both height and width increases. This combined swelling behavior both tightens the annular fibers and widens the footprint. By accomplishing both of these expansion modes the stability and natural functions of the disk are painlessly restored. In brief introduction here, the PDN devices have been constructed of two components: an inner copolymer hydrogel pellet and an outer, superstrong woven jacket of high molecular weight polyethylene (HMWPE) fibers (Fig. 14–1). The PDN-SOLO pellets are formed of a physiologically inert proprietary copolymer of polyacrylonitrile and polyacrylamide, pressure molded into predetermined sizes and shapes, and dehydrated. This controlled dehydration reduces both size and volume while preserving the PDN-SOLO implant’s expansion memory so that when rehydrated inside the prepared, evacuated nucleus cavity, the implant properly fills the cavity (Fig. 14–2). The outer, loosely woven, remarkably strong constraining jacket of HMWPE limits expansion of the pellet thus preventing unrestrained overexpansion that could fracture the end plate. The jacket also makes the insert far easier to surgically manipulate. The presently developed polymer formulation permits the PDN-SOLO device to absorb up to 80% of its dry weight in water, providing a softer, more conformal device while maintaining the important expansion and lifting forces. No other water-soluble hydrogel polymer, among several tested, was found to have these needed abilities. This rehydration-expansion, beginning immediately after insertion and slowing exponentially over 7 to 10 days, restores height and mechanical stability to the disk while maintaining reasonable segmental flexibility. Small, short, platinum-iridium wires are inserted into each end of the pellets or jackets rendering visible the position of an implant by ordinary C-arm fluoroscopic or plain x-ray views (Fig. 14–3). Figure 14–1 Photograph of the PDN-SOLO implant showing the internal copolymeric pellet (lower figure) and the intact implant with its woven jacket of high molecular weight polyethylene. The arrows at the ends of the pellet indicate the internal location of the x-ray-visible platinum-iridium wire stubs. Extensive bench and animal testing were performed on the polymer formulations and jackets using established scientific test methods and in keeping with FDA guidelines for implant materials.7 No intact polymeric device has ever been approved for permanent human intradiskal use; therefore, considerable fundamental investigations were required. The PDN devices and individual components passed all FDAand ISO-required animal, cytological, and toxicity testing. Extensive bench testing was performed at the Raymedica, Inc., facility, and in vitro biomechanical testing using human cadaver spines adhering to standard procedures has been repeatedly performed at two U.S. and two European academic centers.8,9 These test outcomes certified biological safety and mechanical durability and also guided subsequent prototype improvements. Normal or abnormal human disk nucleus tissue specimens, removed at surgery, were found to be unsuitable models for developing nucleus replacement components because this tissue has hydration and mechanical behaviors unlike any of the several experimental hydrogel polymers tested. The natural hygroscopic glycosaminoglycan tissue of the natural nucleus cannot be physically or chemically reproduced. In addition, for testing purposes, no suitable animal model exists for reasonable investigation of any spinal implant other than for studies of tissue acceptance, carcinogenicity, and biocompatibility. Nonetheless, animal studies were ethically performed in standard, approved experiments using large mongrel dogs, large goats, and transgenic mice as well as in tissue culture preparations. However, as is somehow notwithstanding required by the FDA, a baboon study has also been done. None of these tests showed an adverse response to the components, the pellets and jackets, or intact miniaturized implants (the animal disk space is a fraction of the human in diameter and height). The author implanted two of these animal-sized PDN devices into his own intercostal muscle and after 3 years, on removal, they were found to have caused no reactions or deteriorations. The pellet and jacket were subjected to standard mechanical testing for up to 50 million normal range compression cycles and to 10 million compression-translation cycles; neither method showed deterioration of the pellets or jackets. Following prolonged cyclic tests the terminal burst strength of intact implants exceeded the 6 kN limit of the test machine. Thus structural and biological prolonged-term safety was confirmed. Additionally, in cadaver segment biomechanical studies, enucleated segments showed loss of normal stiffness and, therefore, decreased stability as compared with initially intact segments, but when the PDN-SOLO was inserted and hydrated, normal stiffness-stabilization was reestablished. Clinically, the exclusive use of the PDN-SOLO device version began in 2002 and has clearly demonstrated that this new single implant can perform as well as the initial dual implants but with marked simplification of the procedure and marked reduction in dislodgments. The PDN-SOLO implants are 5,7, or 9 mm in dehydrated heights and each is ~29 mm in length; furthermore, the corners of the jackets are now more rounded than were the dual devices. Importantly, although several artificial disk devices have been proposed and actually patented, few have ever been appropriately studied or applied clinically.10–13
The Raymedica Prosthetic Disk Nucleus
(PDN): Stabilizing the Degenerated
Lumbar Vertebral Segment without
Fusion or Total Disk Replacement
 Anterior, Lateral, and Posterolateral Approaches
Anterior, Lateral, and Posterolateral Approaches
Materials and Methods
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Materials and Methods
Materials and Methods Patient Selection
Patient Selection Posterior Approach
Posterior Approach Postoperative Care
Postoperative Care Results
Results Comments
Comments Conclusion
Conclusion Acknowledgments
Acknowledgments