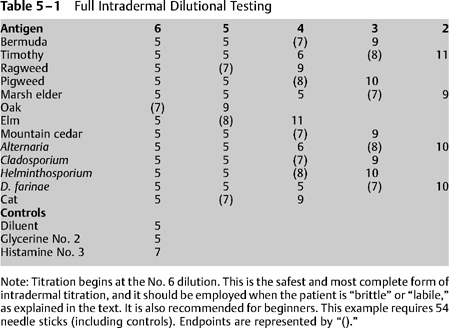
5 The current methodology of IDT varies little from the technique Rinkel1 originally described for skin endpoint titration (SET). The changes that have occurred over the years have been based on observation, experience, and anecdotal material.2 Although the technique described here is recommended as useful, there is no question that scientific validation and investigation of the benefits and drawbacks of IDT are desirable. Other chapters have detailed the required equipment and personnel necessary for performing IDT, as well as the method by which antigens may be chosen. As with any medical procedure, some preparation and planning is required before initiation of testing. First, the value of appropriate education and training cannot be overemphasized. Everyone involved in allergy skin testing must be appropriately trained to prevent, recognize, and treat any of the adverse consequences that may result from that testing, including anaphylaxis. Further, prior to initiation of IDT, an explanation of the procedure, its risks, and its benefits should be provided for the patient and documented with a consent form. The tester can wear nonsterile gloves, or may choose not to do so, but the option should be made available. As a practical matter, most allergy assistants seem to feel some clumsiness when wearing gloves, whereas others appreciate the perception of safety. Although patients should be counseled to avoid drugs that might interfere with skin whealing (as discussed in Chapter 4), the application of controls should precede full skin testing. To ensure that the potential for a whealing reaction exists, the application of a histamine control is necessary. If this positive control wheal fails to grow as expected, further skin testing should be aborted at that sitting. A common reason for such an occurrence is the recent ingestion of an antihistamine or other substance that suppresses whealing. Further skin testing will not be valid until that suppression has worn off.3 The positive control most often used is histamine, at a concentration of ~0.004 mg/mL. This dilution is made by making three fivefold dilutions of a stock solution of histamine, 2.75 mg/5 mL. An intradermal wheal of 4-mm diameter is raised using this histamine control, and the diameter of the wheal is measured after 10 to 15 minutes. A positive response is represented by enlargement of the wheal to a diameter of 7 mm or more. There is no particular significance to a histamine control that enlarges to a greater diameter, but failure to produce a “positive” wheal indicates the need to further investigate the integrity of the whealing response. The skin of some individuals will react to physical trauma alone, raising the potential for a false-positive response during the process of allergy testing. It is therefore necessary to screen for these individuals, called dermatographs, by applying a 4-mm wheal of diluent, which is referred to as a negative control. In most cases the diluent consists of phenolated saline. The wheal resulting from the application of a negative control should not enlarge beyond a 5-mm diameter at 10 to 15 minutes. If the wheal enlarges further, it is likely that false-positive skin tests will result from further testing, rendering the patient a poor candidate for this method of testing. In this situation, in vitro allergy testing will be helpful. At sufficiently concentrated levels glycerin can cause local cutaneous reactions that can also be falsely interpreted as a positive result.4 The concentrations that hold the most potential to give rise to this reaction are contained within antigens at the No. 1 (10% glycerin) and No. 2 (2% glycerin) concentrations. This must be kept in mind if testing is to be done with antigens at these concentrations. Antigenic concentrates contain 50% glycerin as a preservative. The No. 1 dilution (1 : 100 weight per volume w/v) is made up of a fivefold dilution of this antigenic concentrate and will therefore contain 10% glycerin. The No. 2 dilution (1 : 500 w/ v) is diluted another fivefold and will contain 2% glycerin. It is often necessary to test an otherwise negative patient using the No. 2 dilution of antigen, and if this is the case, it is wise to apply a 2% glycerin control (made by making two fivefold dilutions of a stock solution of 50% glycerin). A positive antigenic response is indicated by enlargement of the antigen test wheal beyond the size of the wheal produced by the glycerin control. Although it is uncommon to test a patient with a No. 1 concentration antigen, the same procedure can be followed in such a case. The intradermal tests are generally applied on the upper arm, in areas identified using marking pens. A corresponding key drawn on a sheet of paper may be especially helpful for the novice. Antigens are applied beginning with an anticipated nonreacting concentration. In most cases, this is the No. 6 dilution of the antigen. Approximately 0.05 mL of the test solution should be drawn into a testing syringe. Note that the amount drawn up is immaterial and simply represents a convenient amount that is both sufficient in volume and provides for ease of use. Testing syringes designed specifically for the purposes of intradermal testing are available. These units typically consist of a needle with a short bevel attached directly to the syringe as a single unit, and are intended for increased accuracy of measurement and ease of use while performing skin tests. Prepare the skin with alcohol, then grasp the upper arm with the nondominant hand and draw the skin taut. Brace the heel of the dominant hand on the patient’s arm. Hold the syringe with the thumb and second finger, very much like a dart. The index finger rests lightly on the plunger. Introduce the needle, with the bevel down, just beneath the skin. Some experienced testers then elevate the tip of the needle slightly, resulting in a mild “tenting” of the patient’s skin to aid in delivery of the injection. Once the needle is in position, aspiration for possible blood vessel penetration is unnecessary, but may be done if desired. Gently depress the plunger to form a wheal of 4-mm diameter. Of note, the volume of antigen containing solution that is usually required to raise a 4-mm wheel is about 0.01 to 0.02 mL, but it is important to remember that it is the size of the wheal that is more important than the precise volume that is delivered. Following formation of the appropriate sized wheal, withdraw the needle and set the timer for 10 minutes. Measure the wheal after it is made. If a wheal is too large or too small, it may be crossed out with a marking pen, and another wheal raised nearby. With practice, it is possible to raise 4-mm wheals without difficulty. Every antigen to be tested is initially placed at the No. 6 dilution, using a new syringe for each antigen and discarding it after use. In theory, the controls should be placed and read, then all No. 6 tests applied and subsequently read, and so forth. As a practical matter, the controls and the No. 6 tests are all placed one after the other. The timer is set after the controls have been applied, and by the time all wheals have been made, only a short wait is necessary before these tests can be read. If the controls confirm the presence of whealing capabilities and a normal response to diluent and glycerin, the test wheals are read and the results charted. If a positive wheal (i.e., one with a diameter exceeding that of the original wheal by at least 2 mm) results from the No. 6 application, it is only necessary to apply a No. 5 dilution to produce a confirming wheal, and titration would be completed. If this concept is not clear, the reader should review Chapter 4 before proceeding. If a negative wheal results from the No. 6 dilution, progressively more concentrated solutions are applied until a positive wheal is obtained. The first positive wheal is then followed by yet one more wheal at the next stronger concentration to produce a confirming wheal. This establishes the endpoint, and titration for that particular antigen is completed. Most clinicians feel that if a positive wheal has not been obtained by the time testing has proceeded to the No. 2 dilution, the test should be considered negative. Their reasoning is that even if a positive wheal results from a test with the No. 1 dilution, it would be impossible to obtain a confirming wheal (because testing never is performed with concentrate material). In addition, there is a school of thought that holds that tests using a very concentrated antigen (such as the 1 : 100 w/v No. 1 extract dilution) may yield a significant number of false-positives. Unfortunately, there is no definitive information to resolve this controversy,4 and it is likely that the clinical significance of testing at this concentration will remain controversial. To complete a formal titration, each antigen is tested with progressively stronger concentrations up to the No. 2 dilution, or until an endpoint wheal, followed by a confirming wheal with the next stronger concentration, is obtained (Table 5-1). The endpoint wheal will be 2 mm or more greater in diameter than a negative wheal. The confirming wheal will be at least 2 mm larger than the endpoint wheal. Recall that a 4-mm wheal will enlarge to 5 mm by physical spreading, so a positive wheal will be 7 mm or greater in diameter. Chapter 4 discusses the normal and abnormal progression of wheals that may be seen.
The Mechanics and Interpretation of IDT
Bradley F. Marple and Richard L. Mabry
Ideally, skin testing should provide two key pieces of information important to the evaluation and treatment of allergic disease. At one level it should identify and confirm the presence of antigen-specific allergic sensitivity. At another level, if performed in a quantitative or semiquantitative manner, it should provide information that will allow determination of the relative sensitivity to a specific allergen (known as an “endpoint”). Although the specific methodology may vary among practitioners, the resulting information should ideally be the same. Intradermal dilutional testing (IDT) constitutes one standardized technique of allergy evaluation, and it appears to be as much or more effective than other types of skin testing in achieving these desired goals.
♦ Preparation for Testing
♦ Application of Controls
Positive Control
Negative Control
Glycerin Control
♦ Application of Antigens
♦ Completing the Titration
The Mechanics and Interpretation of IDT
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree






