Fig. 3.1
Chemical structures of the ceramides found in the human stratum corneum. S Sphingosine, P Phytosphingosine, H 6-Hydroxysphingosine, N non-hydroxy fatty acid, A alpha-hydroxy fatty acid, O ω-hydroxy fatty acid, E esterified, D dihydro. In addition the old nomenclature according to the mobility of the CER in the thin layer chromatography was included for clarification
3.2 Stratum Corneum Lipid Nanostructure Investigated With Neutron Diffraction
3.2.1 Basic Principles of Neutron Diffraction
The SC research comprises many biophysical approaches such as Fourier transform infrared spectroscopy, differential scanning calorimetry, atomic force microscopy or nuclear magnetic resonance spectroscopy. Among those the scattering techniques X-ray and especially neutron diffraction are very potent methods to investigate the structure of isolated SC (Bouwstra et al. 1991) as well as SC model membranes constructed from extracted and synthetically derived SC lipids (Bouwstra et al. 1991, 1996, 1998; Friberg and Osborne 1987; Kuempel et al. 1998; McIntosh 2003; McIntosh et al. 1996). Both neutron and X-ray diffraction are similar techniques, with the exception of the irradiation source. While X-rays primarily interact with the electrons of an atom, the interaction of neutrons with the atomic nucleus is short-ranged. To explain the several advantages of neutron over X-ray diffraction a brief explanation of these methods is necessary.
The technique of neutron diffraction is a versatile method to study the structure and dynamics, which specifically applies to biological samples. Due to their specific properties, neutrons may provide structural insights that are hardly obtained by other techniques, e.g., X-ray or light scattering. As non-charged particles, neutrons are enabled to penetrate matter deeply due to the small probability of interaction (Harroun et al. 2006). In contrast to X-rays, which are scattered by the electron cloud, neutrons interact with the atomic nucleus and are scattered isotropically (Dachs 1978). Hence, while the ability of elements to scatter X-rays increases with the atomic number throughout the periodic table of elements, such a correlation does not exist for neutrons (Cantor and Schimmel 1980). Particularly hydrogen, a light atom that is almost invisible for X-rays, is a strong scatterer for neutrons (see Fig. 3.2).
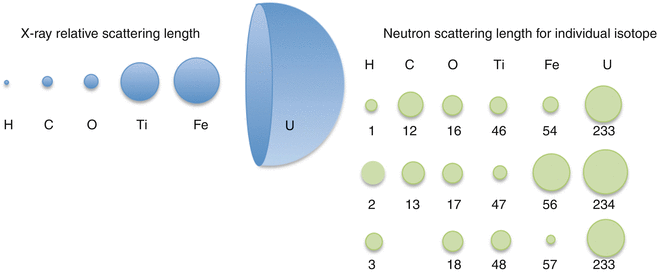

Fig. 3.2
Schematic comparison of the X-ray relative scattering lengths and neutron scattering lengths of different elements and their isotopes
This makes neutron diffraction a particular valuable instrument to investigate structural and dynamic features especially in biological samples, which are rich in hydrogen. Moreover, neutrons show isotope sensitivity, i.e., even different isotopes of one element may have different scattering power for neutrons, for which hydrogen (1H) and deuterium (2H, D) are the most prominent examples (see Fig. 3.2) (Dachs 1978). The possibility to distinguish between components differing in their ability to scatter neutrons in one single sample, the so-called neutron contrast, is of great advantage for the study of biological systems like lipids and proteins (Büldt et al. 1978; Gutberlet et al. 2001; Tomita et al. 1999).
In a typical scattering experiment, a well-collimated neutron beam with a defined wavelength λ irradiates a sample, whereby the neutrons are scattered in all directions depending on the interactions between the sample material and the neutrons. The incoming neutrons interact with the sample and thereby experience a change in their momentum, which appears as a change of neutron direction and/or velocity. Consequently, monitoring the alterations of the neutron’s momentum provides information regarding the structure and dynamics of the sample matter. To describe the change in momentum, the so-called momentum transfer vector, or scattering vector 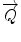 , was introduced and is defined as the difference between incoming
, was introduced and is defined as the difference between incoming 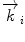 and scattered
and scattered 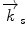 wave vectors
wave vectors 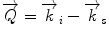 . In addition to a change in direction, the magnitude of
. In addition to a change in direction, the magnitude of 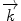 can also change as energy is transferred between incident neutrons and sample. When no energy is conveyed, the scattering process is considered to be totally elastic; therefore,
can also change as energy is transferred between incident neutrons and sample. When no energy is conveyed, the scattering process is considered to be totally elastic; therefore, 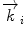 has to be equal to
has to be equal to 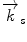 . Taking this in account, the scattering vector
. Taking this in account, the scattering vector 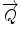 can be evaluated as
can be evaluated as 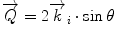 , including the Bragg angle, which in case of crystalline and lamellar material appears at
, including the Bragg angle, which in case of crystalline and lamellar material appears at 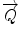 values equivalent to the reciprocal spacing of the lattice:
values equivalent to the reciprocal spacing of the lattice: 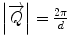 , where by d denotes the characteristic spacing of a set of crystal planes. The complete Bragg formula λ = 2d/sin θ can be received when the wave vector
, where by d denotes the characteristic spacing of a set of crystal planes. The complete Bragg formula λ = 2d/sin θ can be received when the wave vector 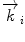 is appropriately substituted with
is appropriately substituted with 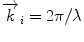 .
.
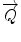 , was introduced and is defined as the difference between incoming
, was introduced and is defined as the difference between incoming 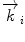 and scattered
and scattered 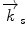 wave vectors
wave vectors 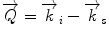 . In addition to a change in direction, the magnitude of
. In addition to a change in direction, the magnitude of 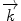 can also change as energy is transferred between incident neutrons and sample. When no energy is conveyed, the scattering process is considered to be totally elastic; therefore,
can also change as energy is transferred between incident neutrons and sample. When no energy is conveyed, the scattering process is considered to be totally elastic; therefore, 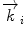 has to be equal to
has to be equal to 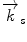 . Taking this in account, the scattering vector
. Taking this in account, the scattering vector 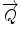 can be evaluated as
can be evaluated as 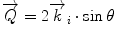 , including the Bragg angle, which in case of crystalline and lamellar material appears at
, including the Bragg angle, which in case of crystalline and lamellar material appears at 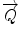 values equivalent to the reciprocal spacing of the lattice:
values equivalent to the reciprocal spacing of the lattice: 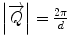 , where by d denotes the characteristic spacing of a set of crystal planes. The complete Bragg formula λ = 2d/sin θ can be received when the wave vector
, where by d denotes the characteristic spacing of a set of crystal planes. The complete Bragg formula λ = 2d/sin θ can be received when the wave vector 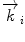 is appropriately substituted with
is appropriately substituted with 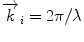 .
.Diffraction can be considered as a special type of scattering, whereby an organized structure such as a crystal or a lamellar arrangement is analyzed. According to Bragg’s law, the incident beams are diffracted at a defined angle 2θ, and due to the interference between the waves, scattered from the parallel planes, diffraction occurs as depicted in Fig. 3.3.
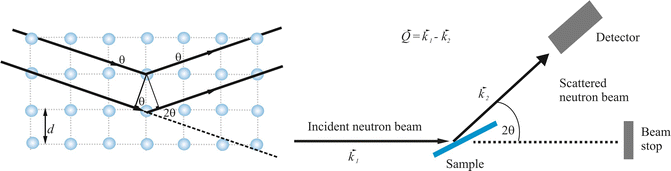

Fig. 3.3
Schematic drawing of the scattering process from ordered material. (Left) Neutrons strike an array of atoms (spheres) from the left side and are scattered to the right. The planes of atoms are separated by the interplanar distance d. The angle θ to the plans of atoms of the incident and the scattered beam are identical. The path length difference between the waves interfering constructively is equal to 2d sin θ. (Right) A typical experimental setup of a neutron diffraction experiment, whereby 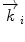 designates the incoming wave vector, while
designates the incoming wave vector, while 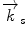 represents the scattered neutron wave vector
represents the scattered neutron wave vector
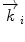 designates the incoming wave vector, while
designates the incoming wave vector, while 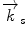 represents the scattered neutron wave vector
represents the scattered neutron wave vectorThe neutron scattering experiment now measures the scattering intensity I as a function of the scattering direction; the interpretation of the data offers information about the structure of the analyzed sample.
3.2.2 Investigation of Stratum Corneum Lipid Model Membranes with Neutron Diffraction
The initial biological materials, analyzed with neutron diffraction, were phospholipids due to the ability to form stable and highly organized multilamellar lipid bilayers necessary for neutron diffraction experiments. In recent years this technique has also been successfully introduced into the elucidation of the structural arrangement of the stratum corneum lipids. The application of neutron diffraction offers new possibilities to investigate the nanostructure of SC lipid systems and especially to gain information about the impact of the different CER subspecies as shown by Charalambopoulou and coworkers on fully hydrated human SC (Charalambopoulou et al. 2002) and by Kiselev et al. on well-defined SC lipid model membranes (Kiselev et al. 2005).
3.2.2.1 Evaluation of the Neutron Diffraction Data
As mentioned above, the scattering process is assumed as an elastic event with no energy transfer taking place. Consequently, the scattering vector 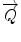 can be correlated to the scattering angle 2θ. Furthermore, the intensity of the scattered neutron is measured as a function of the scattering angle 2θ. As the Bragg condition is complied, the integrated intensities can be calculated by using Gaussian fits to the received Bragg reflections. In order to gain deeper insight into the nanostructural arrangement of the SC lipids, it is necessary to compute the absolute value of the structure factors F h from the integrated peak intensities:
can be correlated to the scattering angle 2θ. Furthermore, the intensity of the scattered neutron is measured as a function of the scattering angle 2θ. As the Bragg condition is complied, the integrated intensities can be calculated by using Gaussian fits to the received Bragg reflections. In order to gain deeper insight into the nanostructural arrangement of the SC lipids, it is necessary to compute the absolute value of the structure factors F h from the integrated peak intensities: 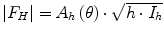 with Lorentz correction h and absorption factor A h (θ) (Franks and Lieb 1979). The structure factor F h serves as a mathematical description in which mode the incoming neutron wave is scattered by the investigated material (Franks and Lieb 1979; Nagle and Tristram-Nagle 2000a, b). The SC lipid multilamellar layers are composed of numerous bimolecular lipid membranes, which in other terms can be described as two equal monolayers facing each other. Such stacks of lipid layers are considered centrosymmetric for the neutron diffraction experiment, which allows for the construction of the neutron scattering length density (NSLD) profile ρ s(x) across the bilayer as Fourier transform
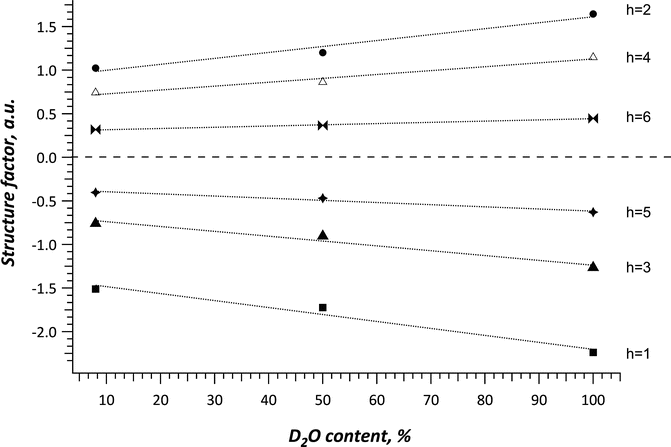
with Lorentz correction h and absorption factor A h (θ) (Franks and Lieb 1979). The structure factor F h serves as a mathematical description in which mode the incoming neutron wave is scattered by the investigated material (Franks and Lieb 1979; Nagle and Tristram-Nagle 2000a, b). The SC lipid multilamellar layers are composed of numerous bimolecular lipid membranes, which in other terms can be described as two equal monolayers facing each other. Such stacks of lipid layers are considered centrosymmetric for the neutron diffraction experiment, which allows for the construction of the neutron scattering length density (NSLD) profile ρ s(x) across the bilayer as Fourier transform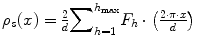 (Nagle and Tristram-Nagle 2000b). In order to calculate the NSLD profile, it is essential to define the sign of the structure factor F h. This can be easily done by variation of the D2O/H2O ratio in the surrounding atmosphere, the so-called contrast variation (Wiener and White 1991), assuming that water can penetrate between the bilayer sheets (Franks and Lieb 1979; Worcester 1976). It was shown for such symmetrical and hydrated bilayers that the phase problem of F h s simplifies to the determination of the sign of + or – (Franks and Lieb 1979) and can be derived from the slope of the correlation of F h against the D2O content in water vapor as shown in Fig. 3.4
(Nagle and Tristram-Nagle 2000b). In order to calculate the NSLD profile, it is essential to define the sign of the structure factor F h. This can be easily done by variation of the D2O/H2O ratio in the surrounding atmosphere, the so-called contrast variation (Wiener and White 1991), assuming that water can penetrate between the bilayer sheets (Franks and Lieb 1979; Worcester 1976). It was shown for such symmetrical and hydrated bilayers that the phase problem of F h s simplifies to the determination of the sign of + or – (Franks and Lieb 1979) and can be derived from the slope of the correlation of F h against the D2O content in water vapor as shown in Fig. 3.4
.
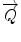 can be correlated to the scattering angle 2θ. Furthermore, the intensity of the scattered neutron is measured as a function of the scattering angle 2θ. As the Bragg condition is complied, the integrated intensities can be calculated by using Gaussian fits to the received Bragg reflections. In order to gain deeper insight into the nanostructural arrangement of the SC lipids, it is necessary to compute the absolute value of the structure factors F h from the integrated peak intensities:
can be correlated to the scattering angle 2θ. Furthermore, the intensity of the scattered neutron is measured as a function of the scattering angle 2θ. As the Bragg condition is complied, the integrated intensities can be calculated by using Gaussian fits to the received Bragg reflections. In order to gain deeper insight into the nanostructural arrangement of the SC lipids, it is necessary to compute the absolute value of the structure factors F h from the integrated peak intensities: 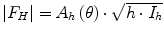 with Lorentz correction h and absorption factor A h (θ) (Franks and Lieb 1979). The structure factor F h serves as a mathematical description in which mode the incoming neutron wave is scattered by the investigated material (Franks and Lieb 1979; Nagle and Tristram-Nagle 2000a, b). The SC lipid multilamellar layers are composed of numerous bimolecular lipid membranes, which in other terms can be described as two equal monolayers facing each other. Such stacks of lipid layers are considered centrosymmetric for the neutron diffraction experiment, which allows for the construction of the neutron scattering length density (NSLD) profile ρ s(x) across the bilayer as Fourier transform
with Lorentz correction h and absorption factor A h (θ) (Franks and Lieb 1979). The structure factor F h serves as a mathematical description in which mode the incoming neutron wave is scattered by the investigated material (Franks and Lieb 1979; Nagle and Tristram-Nagle 2000a, b). The SC lipid multilamellar layers are composed of numerous bimolecular lipid membranes, which in other terms can be described as two equal monolayers facing each other. Such stacks of lipid layers are considered centrosymmetric for the neutron diffraction experiment, which allows for the construction of the neutron scattering length density (NSLD) profile ρ s(x) across the bilayer as Fourier transform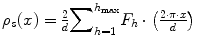 (Nagle and Tristram-Nagle 2000b). In order to calculate the NSLD profile, it is essential to define the sign of the structure factor F h. This can be easily done by variation of the D2O/H2O ratio in the surrounding atmosphere, the so-called contrast variation (Wiener and White 1991), assuming that water can penetrate between the bilayer sheets (Franks and Lieb 1979; Worcester 1976). It was shown for such symmetrical and hydrated bilayers that the phase problem of F h s simplifies to the determination of the sign of + or – (Franks and Lieb 1979) and can be derived from the slope of the correlation of F h against the D2O content in water vapor as shown in Fig. 3.4
(Nagle and Tristram-Nagle 2000b). In order to calculate the NSLD profile, it is essential to define the sign of the structure factor F h. This can be easily done by variation of the D2O/H2O ratio in the surrounding atmosphere, the so-called contrast variation (Wiener and White 1991), assuming that water can penetrate between the bilayer sheets (Franks and Lieb 1979; Worcester 1976). It was shown for such symmetrical and hydrated bilayers that the phase problem of F h s simplifies to the determination of the sign of + or – (Franks and Lieb 1979) and can be derived from the slope of the correlation of F h against the D2O content in water vapor as shown in Fig. 3.4
Fig. 3.4
Illustration of the dependency of the membrane structure factor Fh of the orders h = 1, 2, 3, 4 and 5 on the D2O content on the water vapor a SC lipid model system
The NSLD profile offers detailed information about the nanostructure of the investigated lipid membrane and can also contribute to assign the position and orientation of the bilayer constituents. Furthermore, the evaluation of the NSLD profile allows for determining specific membrane regions such as the polar head groups, CH3 groups, hydrocarbon chain region, and the region of cholesterol location (Kiselev et al. 2005). Next to the assignment of the position of these groups in the lipid bilayer membrane, the determination of parameters such as the region of polar head group or thickness of the intermembrane space further improves and intensifies the knowledge about such SC lipid organization.
3.2.3 Advantages and Disadvantages of Neutron Diffraction
As up to now a clear and detailed picture of the organization of the SC lipid matrix on a molecular scale has not been elucidated, it is of supreme importance to comprehend the mode of action of the different SC lipid classes and particular the impact of the different ceramide subspecies. For the investigation of the driving forces and mechanisms that govern the self-assembling process of such lipid layers, the native SC lipid membranes are too complex objects to probe, especially with neutron diffraction. Consequently, for such an approach, model membranes will be the objects of choice. Moreover, issues due to the variability of the native lipids, for example, the variability in the head group architecture, can be overcome.
Neutrons as irradiation source are non-charged particles; they have only small interaction potential with matter, which enables a deep penetration into the studied material. This makes this method particularly suitable for biological issues such as the investigation of the structural arrangement of the SC lipids. In addition, as mentioned before the neutrons are scattered differently by different isotopes of the same element (see Fig. 3.2). This special feature renders the possibility of the contrast variation as described before. In the same line, there is another distinct advantage of the neutron diffraction technique, which is the possibility of specific deuteration, as the coherent scattering length b coh (the scattering ability) of hydrogen (1H) and its isotope deuterium (2H) differs significantly (b coh(1H) = − 3.741 fm, b coh(2H) = 6.671 fm). Accordingly, hydrogen atoms in a lipid molecule can be specifically substituted by deuterium, which does not alter the properties of the lipid molecule. When a partially deuterated lipid sample is compared to its protonated counterpart, it is possible to identify the exact position of the labeled group within the lipid membrane (see Fig. 3.5). This is a distinct advantage of neutron diffraction over X-ray diffraction, which does not allow for such localization. As the SC lipid molecules are rich in hydrogen atoms, there is a variety of substitution positions, which then permit to make distinction between different conformational states of the studied lipid species. This feature is of high interest, especially for the investigation of SC lipids, as it is known for CER to exhibit different conformational states (Dahlen and Pascher 1972, 1979; Raudenkolb et al. 2003a, b, 2005).
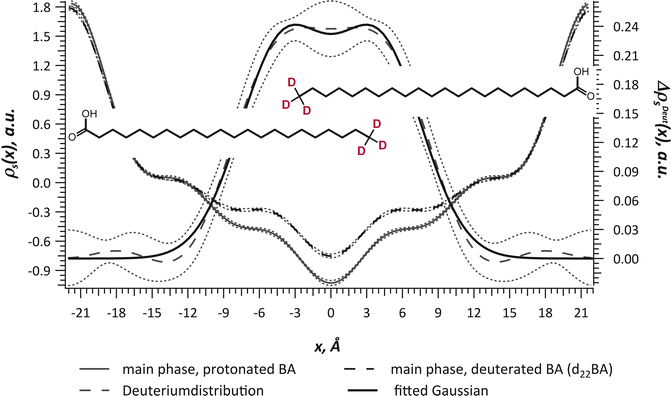

Fig. 3.5
Example of the localization of partially deuterated behenic acid (BA) molecules (d22BA) in a SC lipid model membrane composed of ceramide [AP], cholesterol, d22BA and cholesterol sulfate. The neutron scattering length density (NSLD) profiles display the comparison of the sample membrane containing either deuterated (dashed line) or protonated BA (solid line). Dotted lines: corresponding errors, Long dash: difference NSLD profile, Fat solid line: fit of the difference NSLD profile by two Gaussian functions (deuterium distribution). All measurements were carried out at 57 % relative humidity, at 8 % D2O in water vapor and T = 20 °C
The disadvantages are the limiting factors for application of the neutron diffraction for exploration of the SC lipid matrix. When compared to X-rays the neutron fluxes are relatively small, which necessitates a much longer experimental timescale and a higher amount of lipid material to achieve a reasonable signal-to-noise ratio. Furthermore, when native SC is probed with neutron diffraction, this yields to only one or two diffraction orders, which are not sufficient for the analysis of the NSLD profile (Charalambopoulou et al. 2002). Consequently only SC lipid model systems can be studied.
Another drawback of the neutron diffraction technique is its availability, as there exist only a few neutron facilities at which such an experiment can be carried out (e.g., Helmholtz Centre Berlin for Material and Energy (HZB) and Institut Laue-Langevin (ILL), Grenoble, France).
3.2.4 X-Ray Diffraction for the Investigation of the Stratum Corneum Lipids
As mentioned before, X-ray diffraction has been widely used for the investigation of the structural arrangement of the SC lipids. So Hatta and coworkers could establish the impact of ethanol on the lipid membranes. They discovered by X-ray diffraction, that lipid compounds can be extracted and even recrystallized as well (Hatta et al. 2001). The investigations of Kessner et al. could establish by X-rays that the phase behavior of the long-chain CERs is effected by their long acyl rests (Kessner et al. 2010).
Compared to other scattering techniques, it has a variety of advantages. In contrast to the above-described neutron diffraction technique, X-rays are scattered by the electrons surrounding the atomic nuclei. This results in peaks of high intensity and high resolution. Furthermore, many X-ray sources for such experiments are accessible.
Nevertheless, as described above the major drawback of the application of X-ray in the field of SC research is the low capability to depict light atoms such as hydrogen, which is one of the main components of a biological relevant material. Furthermore, as the number of electrons between different isotopes of the same element does not change, X-rays cannot distinguish isotopes. Its application as an irradiation source does not allow for the evaluation of the sign of the structure factor, which is essential in order to be able to calculate the scattering length density profile to gain deeper insight into the arrangement of the lipid bilayer. When the lamellar thickness of a lipid membrane is changed by way of varying the thickness of the water layer, it is possible to evaluate the sign of the structure factor and consequently calculate the electron density profile. However, it is well known that the repeat distances of SC lipid mixtures prepared from ceramides (CER), cholesterol (CHOL), and free fatty acids (FFAs) are very insensitive to hydration and that especially for the short periodicity phase (SPP), only a limited number of diffraction orders are obtained with X-ray diffraction. Therefore, again it is difficult to acquire an electron density profile by X-ray diffraction analysis.
3.3 ω-acyl Chain Ceramides and Their Influence on the Nanostructure of the Stratum Corneum Lipid Matrix
The lipids of the SC and particularly the ceramides (CER) are responsible to uphold the proper barrier function as pointed out in the introduction. The CER are a very heterogeneous group of sphingolipids (see Fig. 3.1), which can roughly be divided into two groups: (1) short-chain CER such as CER[AP] or CER[NP] and (2) the exceptionally long-chain ω-acyl CER such as CER[EOS] or CER[EOP]. So far, there has been a general consensus that especially the long-chain ω-acyl CER seem to be of particular relevance because of their unique structure.
Besides the assumed importance of long-chain ω-acyl CER in forming the so-called long-periodicity phase (LPP), their presence plays a key role with regard to some skin diseases. For atopic dermatitis there was found a decrease especially in the CER[EOS] content as proposed by Yamamoto and coworkers (Yamamoto et al. 1991), whereas psoriatic skin among others is thought to be caused by an increase in the CER[EOS] amount (Motta et al. 1994).
3.3.1 Physicochemical Aspects
In addition to the amidation of the sphingosine backbone, the ω-hydroxylated fatty acid is at its ω-position mainly esterified with a linoleic acid (EO) (Coderch et al. 2003). Nevertheless, Hinder and coworkers identified for CER [EOS] different amid-bound fatty acids with chain lengths varying from 17 to 22 carbon atoms (Hinder et al. 2011). The same chain length ranging was found for the sphingosine part. Therefore, it was concluded that not only linoleic acid as fatty acid compound is esterified to the sphingosine backbone. Subsequently, the influence of these different chain lengths was studied by de Sousa Neto et al. with small-angle X-ray scattering and Fourier transform infrared spectroscopy (de Sousa Neto et al. 2011). Their results show an important influence of chain length on the lipid organization. Whereas linoleate- and oleate-linked fatty acid CER[EOS] showed both the formation of the LPP and SPP, the stearate-linked variety did not form the LPP. Hence, unsaturated amid-bound fatty acids seem to be crucial for the formation of the LPP.
Due to the high mobility of the exceptionally long-chain ω-acyl residue, these CER form less ordered structures than it is known for short-chain CER (Raudenkolb 2002). Additionally, the melting points being 86 °C for CER[EOS] and above 100 °C for CER[EOP], respectively, hardly differ from those found for short-chain CER as stated by Kessner and coworkers (Kessner et al. 2010). The discrepancy in melting points of both ω-acyl chain CER was argued to be due to the more polar head group structure of CER[EOP] (2 OH groups for CER[EOS] versus 3 OH groups for CER[EOP]), which enables this CER to create more hydrogen bonds. In contrast to their expectations, Kessner and coworkers could additionally ascertain that the thermotropic phase behavior of these CER is reversible and the melting process does not induce major modifications of the lipids (Kessner et al. 2010). This is in line with other discoveries, which found the polar head group architecture to be responsible for polymorphism (Raudenkolb et al. 2003a, b, 2005).
3.3.2 Long-Chain ω-acyl Ceramides Studied Using Native Stratum Corneum
As there is a high demand to comprehend the structural arrangement of the different components of the SC lipid, especially, the long-chain CER were supposed to be of great influence. Consequently, the focus of many researchers was first placed on these exceptionally long-chain ω-acyl CER with their extraordinary characteristics concerning SC lipid organization. For example, Bouwstra et al. stated the importance of CER[EOS] not only for the formation of the LPP but also for the formation of a liquid phase which enables molecules to permeate along the lipid layer (Bouwstra et al. 2002). In this context the same group analyzed mixtures of CER, cholesterol (CHOL), and free fatty acids (FFAs) with regard to a diminishing content of CER[EOS] by X-ray and electron diffraction studies (Bouwstra et al. 2001a). According to their assumption, the fraction of lipids forming the LPP decreases by reducing the amount of CER[EOS]. Thus, they concluded that the presence of the long-chain ω-acyl CER is necessary for the formation of the LPP and subsequently for a proper barrier function of the SC lipid matrix. Resulting from different electron diffraction studies, the LPP is described as a trilamellar broad-narrow-broad arrangement of the SC lipids with a membrane thickness or repeat distance of 13 nm (Madison et al. 1987; White et al. 1988). Based on these insights Bouwstra et al. developed the sandwich model, depicted in Fig. 3.6. According to this model the liquid sublattice consists of CHOL and the linoleic acid residues of the long ω-acyl CER[EOS], [EOP], and [EOH], which are located in the center of this trilamellar structure and encompass nearly 3 nm of the total 13 nm thickness of the LPP. The adjacent crystalline phases with a broadness of 5 nm on either side are composed of long saturated hydrocarbon chains of the CER and FFA (Bouwstra et al. 2001a). Except the research of McIntosh et al. (1996), in which isolated CER from native pig epidermis were employed, all studies mentioned above directly used native SC derived from a pig or mouse tissue for their investigations.
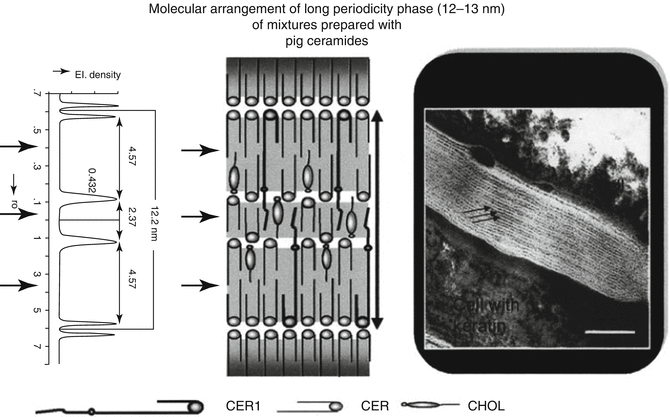

Fig. 3.6
Schematic presentation of the molecular arrangement of the long-periodicity phase (LPP). Reprinted from Bouwstra et al. (2001a) with permission from Karger Publisher
But as pig CER differ structurally from CER originated from human tissue, Bouwstra and coworkers compared their previous results of mixtures containing pig CER with those containing human CER (Bouwstra et al. 2001b). Similar to pig CER/CHOL mixtures, human CER/CHOL mixtures showed the formation of the LPP with the difference, that by addition of FFA the LPP disappeared and has been replaced by a short periodicity phase (SPP) (Bouwstra et al. 2001b). Contrary to these findings various work groups could not detect an LPP by cryoelectron microscopy (Al-Amoudi et al. 2005; Pfeiffer et al. 2000) and X-ray diffraction (Garson et al. 1991) in human SC. They explained the discovery of the LPP in former researches as an artifact due to the fixation with Ruthenium tetroxide. Ruthenium tetroxide is necessary for the electron diffraction and might yield to the misinterpretation of the received data. However, the fixation problem does not account for the X-ray diffraction results.
3.3.3 Synthetically Constructed Long-Chain ω-acyl Ceramides
There are several disadvantages when native SC lipids are used. For instance, the variability in chain length of either the FFA or the CER-bound fatty acids and differences in the CER head group architecture (see Fig. 3.1) can circumvent the assignment of different characteristics to individual lipids, especially the CER subclasses. In recent years synthetically constructed CER with defined chemical structures have been introduced into the SC lipid research, enabling a more reliable transduction of the physicochemical behavior to the structural characteristics of special CER.
This was taken into consideration by de Jager et al. (2004), who confirmed the existence of the LPP by investigating synthetic CER in mixtures with CHOL and FFA using small-angle X-ray diffraction. Additionally, they concluded that there is a close connection with the formation of the LPP and the presence of CER[EOS]. They stated that, while partial replacement of CER[EOS] by CER[EOP] does not influence phase behavior, complete substitution leads to a phase separation of CER[EOP] and a reduction of the LPP.
Another study performed by Kessner and coworkers also employed synthetically derived CER and applied both X-ray diffraction and Fourier transform Raman spectroscopy in order to ascertain these findings. Contrary to the previously described investigation, the physicochemical behavior of only the synthetic long ω-acyl CER[EOS] and [EOP] (Kessner et al. 2010) was studied without FFA or CHOL. These investigations revealed remarkable insights in this regard. While CER[EOS] in dry state only arranges in a SPP, CER[EOP] already forms the LPP. It was deduced that the differences in the head group architectures are responsible as this is the only dissimilarity between both CER species. They argued that the additional hydroxyl group in CER[EOP] is responsible for more hydrogen bonds and therefore enables the formation of a high-ordered package preventing the ω-acyl chains extending into the adjacent bilayer. Once both CER are hydrated, they are able to form the LPP with a repeat distance of 12 nm (Kessner et al. 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree