Chapter 4 The Cosmeceutical Marketplace
EFFICACY
Efficacy is evaluated in numerous ways. There are two primary categories of measurement for ‘antiaging’ moisturizers: ‘health’ benefits (how good a moisturizer it is) and ‘beauty’ benefits (its effect on appearance of fine lines and wrinkles, skin tone and texture, etc.). For ‘health’ benefits, transepidermal water loss (TEWL) can be measured objectively, as can capacitance, an indirect measure with a correlation to moisturizer efficacy. Expert grading is carried out to evaluate visual dryness and redness.
 Is the product from a reputable company? As a general rule, large companies have much more stringent self-regulation on claims than smaller companies.
Is the product from a reputable company? As a general rule, large companies have much more stringent self-regulation on claims than smaller companies.
 Is proof offered via some professional interchange? If the manufacturer will not invest in a clinical study, rule out the product. It is possible, but unlikely, that a manufacturer with a great product just chooses not to prove it.
Is proof offered via some professional interchange? If the manufacturer will not invest in a clinical study, rule out the product. It is possible, but unlikely, that a manufacturer with a great product just chooses not to prove it.
 What is the quality of the proof? The manufacturer’s dilemma is that opportunities to profit from sales of new technology occur in the here and now, and publishing in some journals happens ‘in the future’, if at all. If the manufacturer has made reasonable attempts to do quality studies and has exposed them to peer review in some manner, they should be viewed favorably (posters at medical meetings, or engaging reputable dermatologists in the design and implementation of a clinical study would qualify). The term ‘data on file’ means a study was done, usually by the manufacturer, but not published, and so there is no citation to offer. If the reputation of the manufacturer is good, then this represents quality research.
What is the quality of the proof? The manufacturer’s dilemma is that opportunities to profit from sales of new technology occur in the here and now, and publishing in some journals happens ‘in the future’, if at all. If the manufacturer has made reasonable attempts to do quality studies and has exposed them to peer review in some manner, they should be viewed favorably (posters at medical meetings, or engaging reputable dermatologists in the design and implementation of a clinical study would qualify). The term ‘data on file’ means a study was done, usually by the manufacturer, but not published, and so there is no citation to offer. If the reputation of the manufacturer is good, then this represents quality research.
COMPLIANCE
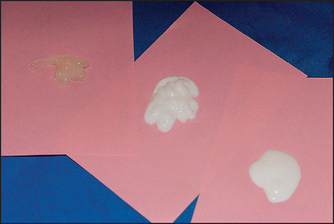
Compliance in the category of cosmeceuticals is most often achieved when the final product is aesthetically pleasing and can be considered overall as a cosmetically elegant product (Fig. 4.1). Such attributes as having superior packaging, a nice feel and spread during application, absorbing readily into the skin, and improving the appearance of the skin immediately in a cosmetic or superficial way are all important when it comes to product usage compliance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree