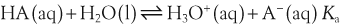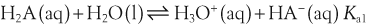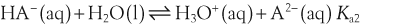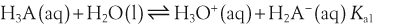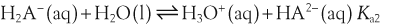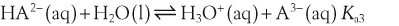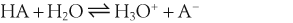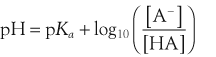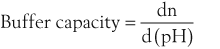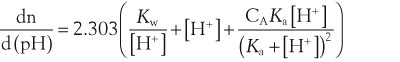1 The Chemistry of Peels
A Hypothesis of Action Mechanisms and a Proposal of a New Classification of Chemical Peelings
Useful Elements of Basic Chemistry
• Acids
Chemicals or substances having the property of an acid are said to be acidic (adjective).
• Polarity and the inductive effect
The polarity of the H—A bond is the first factor contributing to the acid strength.
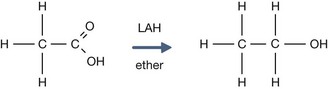
The pKa for ethanol is 16, compared to 4.76 for acetic acid.
• Atomic radius and bond strength
The size of the atom A or atomic radius is the second factor contributing to the acid strength.
• Chemical characteristics
• Buffer solution
Le Chatelier’s Principle
In a solution there is an equilibrium between a weak acid, HA, and its conjugate base, A−:
Henderson–Hasselbach Equation
The acid dissociation constant for a weak acid, HA, is defined as:
One should remember that the calculated pH may be different from measured pH.
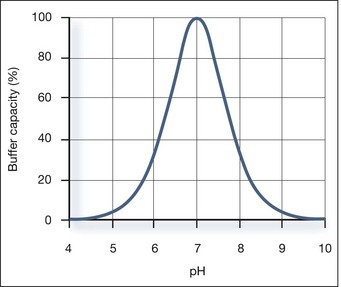
Buffer Capacity
Buffer capacity is a quantitative measure of the resistance of a buffer solution to pH change on addition of hydroxide ions. It can be defined as follows:
where dn is an infinitesimal amount of added base and d(pH) is the resulting infinitesimal change in pH. With this definition the buffer capacity can be expressed as:
where Kw is the self-ionization constant of water and CA is the analytical concentration of the acid, equal to [HA]+[A−]. The term Kw/[H+] becomes significant at pH greater than about 11.5 and the second term becomes significant at pH less than about 2. Both these terms are properties of water and are independent of the weak acid. Considering the third term, it follows that:
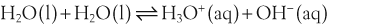
 H+ + A−, where HA represents the acid and A− is the conjugate base. Acid–base conjugate pairs differ by one proton, and can be interconverted by the addition or removal of a proton (protonation and deprotonation, respectively). Note that the acid can be the charged species and the conjugate base can be neutral in which case the generalized reaction scheme could be written as HA
H+ + A−, where HA represents the acid and A− is the conjugate base. Acid–base conjugate pairs differ by one proton, and can be interconverted by the addition or removal of a proton (protonation and deprotonation, respectively). Note that the acid can be the charged species and the conjugate base can be neutral in which case the generalized reaction scheme could be written as HA 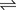 H+ + A. In solution there exists an equilibrium between the acid and its conjugate base. The equilibrium constant K is an expression of the equilibrium concentrations of the molecules or the ions in solution. Brackets indicate concentration, such that [H2O] means the concentration of H2O. The acid dissociation constant Ka is generally used in the context of acid-base reactions. The numerical value of Ka is equal to the concentration of the products divided by the concentration of the reactants, where the reactant is the acid (HA) and the products are the conjugate base and H+.
H+ + A. In solution there exists an equilibrium between the acid and its conjugate base. The equilibrium constant K is an expression of the equilibrium concentrations of the molecules or the ions in solution. Brackets indicate concentration, such that [H2O] means the concentration of H2O. The acid dissociation constant Ka is generally used in the context of acid-base reactions. The numerical value of Ka is equal to the concentration of the products divided by the concentration of the reactants, where the reactant is the acid (HA) and the products are the conjugate base and H+.