Fig. 2.1
Incidence of breast and ovarian cancers in women with hereditary (high-risk), familial (moderate-risk) and sporadic (general population) BC [8, mod with permission]
Sporadic BC (70–75 %) represents the largest fraction of BC. It appears in individuals of the general population, without a significant familiarity, by the action of somatic mutations, mostly unknown. Few somatic mutations have been identified in the HER2 and TP53 genes associated with some cases of BC.
Familial, or family related, BC (15–20 %). In general, family-related cancer is a descriptive term that indicates an aggregation of multiple cases of cancer in the same family (paternal and/or maternal line), without a clear transmission of the disease from one generation to the next or ascertained responsible gene.
However, it is commonly accepted that in some cases the lack of mutation may be due to two main factors: the patient is investigated only in BRCA1 and 2 genes (and not on other susceptibility genes) and international databases of mutation are still extremely poor or plenty of non-informative data.
Hereditary BC (5–10 %) is characterised by ascertained (or highly suspected from the analysis of the pedigree) genetic mutations that are transmitted to descendants. Its inherited susceptibility to BC is established on the basis of an identified germ-line mutation in one allele of a high-penetrance susceptibility gene, such as BRCA1 and BRCA2. Inactivation of the second allele of these tumour suppressor genes would be an early event in the multistep pathway of carcinogenesis.
Ovarian cancer (OC) too could be involved in the same gene mutations of BC. OC is preferably differentiated only between sporadic (90 %) and hereditary (10 %).
Although most cases of BC are not inherited, suspicious hereditary BC should be investigated because the cancer risk depends on the genes involved. This is crucial even if, actually, people inherit an increased risk of cancer, not the disease itself, and not all people who inherit mutations in these genes will develop cancer. In other cases, as in the most family-related BC, the inheritance of BC risk is unclear. However, it can be approximately calculated as a percentage using one of the risk assessment tools, starting from the family tree (pedigree chart).
FIRST SELECTION. The presence of an inherited predisposition to BC and/or OC in a family should be suspected on the basis of the following features:
Incidence significantly higher than expected
Age of onset younger (<35 years) than sporadic cases
Increased frequency of bilateral tumours
Family history of BC in males
Family history of ovarian cancer
Association between breast and ovarian cancer in the same patient in the same family
BC in a young woman with aggressive phenotype (high-grade DCIS, RE / RPG <10, Ki67 >15, triple negative, HER2 overexpression)
WOMEN ELIGIBLE FOR GENETIC TESTING are healthy women as well as women suffering from BC, both with more than 10 % chance of being carriers of mutations in the genes BRCA1/2. Criteria may be different in different countries, but usually all broadly refer to those of the National Comprehensive Cancer Network Guidelines [8, 9] (Fig. 2.2).
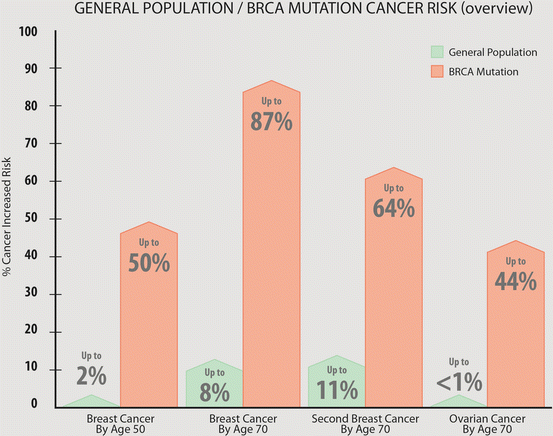

Fig. 2.2
Incidence of breast and/or ovarian cancer in BRCA mutation carriers and in general population [9, mod with permission]
Healthy women eligible for genetic testing are unaffected individual with a family history of one or more of the following:
A known mutation in a BC susceptibility gene within the family
≥2 breast primaries in single individual
≥2 individuals with breast primaries on the same side of the family
≥1 ovarian cancer primary from the same side of the family
First- or second-degree relative with BC ≤45 years
≥1 family member on the same side of family with a combination of BC and ≥1 of some cancer (especially if early onset) as pancreatic cancer, prostate cancer (Gleason score ≥7), sarcoma, adrenocortical carcinoma, brain tumours, endometrial cancer, leukaemia/lymphoma and thyroid cancer
Male BC
Women suffering from BC eligible for genetic testing are individual with one or more of the following:
A known mutation in a BC susceptibility gene within the family
Early-age-onset BC
Triple negative (ER, PR, HER2) BC
Two BC primaries in a single individual
BC at any age and:
≥1 close blood relative with BC ≤50 years or
≥1 close blood relative with epithelial ovarian cancer at any age or
≥2 close blood relatives with BC and/or pancreatic cancer at any age
≥1 family member on the same side of the family with a combination of BC and ≥1 of some cancer (especially if early onset) as pancreatic cancer, prostate cancer (Gleason score ≥7), sarcoma, adrenocortical carcinoma, brain tumours, endometrial cancer, leukaemia/lymphoma and thyroid cancer
Ovarian cancer
Male BC
Other domestic criteria highlight the age of family members at diagnosis, so that an average risk is considered for women who have:
Only 1 first-degree relative diagnosed after 40 years of age or
Only 2 first-degree relatives diagnosed after 60 years of age without any other association (i.e. ovarian cancer, male BC, etc.)
In the same way, women likely to be at moderate risk are those who have:
Two first-degree relatives diagnosed between 50 and 59 years of age
Two second-degree maternal relatives diagnosed before 50 years of age
One first- or second-degree relative diagnosed between 50 and 59 years of age + 1 familiar first/second-degree relative with ovarian cancer at any age without any other association (i.e. male BC, etc.)General Genetic Testing Strategies. Ideally, genetic testing should begin with a member of the family having the highest probability of having a hereditary condition, typically the member with an early-onset cancer. Doing so increases the likelihood of finding the disease‐causing mutation if one can be detected in the family. If the highest risk relative has no detectable mutations, that suggests the disease causing mutation in the family cannot be detected, and therefore, testing other individuals in the family (particularly unaffected individuals) is unlikely to be helpful in the risk assessment. If a mutation has been identified in a blood relative, the consultant can undergo single site testing in order to save on cost.Testing individuals of Ashkenazi Jewish descent should start with testing of the three common Ashkenazi Jewish founder mutations; reflexing to comprehensive testing can be considered depending on the strength of suspicion for HBOC and whether the individual has any non-Ashkenazi Jewish ancestry.Testing unaffected patients younger than age 18 years for BRCA1/BRCA2 is not recommended.
2.3.2 Genetic Counselling
Subjects of genetic testing are:
Counsellor, a specialist in genetic counselling
Consultant, the person who needs the referral and who has never had cancer
Proband, the consultant with BC who is the first subject in a study of a genetic character in a family germ line. The information for the compilation of the family tree is collected starting from the proband. If the consultant is healthy, the proband will be the closest case of tumour in the family tree.
The Phases of Genetic Counselling. Firstly a thorough reconstruction of the family history is done by verification of the available data and of the clinical documentation. Practice for collecting clinical data includes some important recommendations:
Family history can be collected by the specialist in medical genetics.
History should be accurate and must take into account the maternal line and paternal line; each report must record the age at diagnosis of cancer and age of death and must be verified by examination of the documentation or the type of intervention or therapy.
In case of doubt (i.e. uncertainty between benign or malignant tumour in a relative) the subject should be considered negative.
If criteria for eligibility to genetic testing subsist, the next steps are a discussion of potential benefits and limitations of the test and then a complete assessment of the case. The patient is allowed a week to decide whether to consent to the test. After that, the test is performed and the patient is recalled for results delivery and, if the mutation is confirmed, details are discussed.
Pros of genetic testing. In summary, genetic testing can help to:
Understand what is the real risk of developing BC or OC
Implement adequate preventive custom strategy
Participate in programs of intensive surveillance, more close screening than the existing one for the general population
Provide important information to the consultants’ families
Frame with accuracy the risk of cancer, which may mean, taking, for instance, the case of a negative result of the test on a healthy family, that the consultant has the same risk of contracting BC or OC as the general population, etc.
Contribute to research
Cons of genetic testing. Back to front, the disadvantages are:
There is currently no preventive strategy of absolute efficacy to prevent the possibility of developing cancer.
Difficulties may arise in coping with the news of genetic predisposition: anxiety and depression immediately following the notification of the outcome are quite common.
It is a fact that cannot be changed in the course of life.
A negative test result could cause a dangerous sense of security, since a negative result does not mean the consultant risk of contracting BC or OC is zero, but that they have the same risk as the general population.
Reasons why some women wish to undergo the genetic testing are (in brief):
Desire to have an explanation why in their own families there have been several cases of cancer.
Desire to put an end to the uncertainties that create anxiety and worries.
Knowing the risk of developing cancer can help to better design their lives (marriage, pregnancy).
Desire to do something useful for their families.
Feeling of responsibility towards their children.
Reasons why some women refuse the genetic testing are:
Fear of having to live with a risk of cancer, for which there is no decisive preventive intervention
Embarrassment towards family members
Sense of guilt towards their children
2.3.3 Risk Assessment Tools
Some individuals refuse to do the test for various reasons or because it is expensive. In this case BC risk assessment tools could be exploited to predict individual BC risk [10]. There are a number of models available (Gail, Claus, Tyrer-Cuzick, BRCAPRO and BOADICEA models) to assess both BC risk and the chances of identifying a BRCA1/2 mutation. Some models perform both tasks, but none are yet totally discriminatory as to which family has a mutation and who will develop BC. Useful in most cases, they do not give a truthful estimate of risk in some women including those:
With a personal history of invasive BC, ductal carcinoma in situ (DCIS) or lobular carcinoma in situ (LCIS)
With a strong family history of BC, who may have an inherited gene mutation
The Gail model is an accessible interactive computer programme online (http://www.cancer.gov/bcrisktool) that incorporates a number of established risk factors and estimates a woman’s risk of developing invasive BC during the next 5-year period and up to age 90 (lifetime risk). A 5-year risk of 1.67 % or higher is considered elevated. This model is not recommended for use with women having a strong family history since it excludes some well-established factors associated with hereditary BC.
The Claus model provides a more accurate estimate of risk for women with a family history of BC by taking into account both maternal and paternal histories, including second-degree relatives. The model can also incorporate a family history of ovarian cancer. However, unlike the Gail model, the Claus model does not include many of the other risk factors known to increase risk. It may therefore underestimate the risk in women with exposure to certain environmental, behavioural or reproductive factors.
The BRCAPRO is a statistical model, with associated software (http://bcb.dfci.harvard.edu/bayesmendel/software.php), used to estimate the probability of having a BRCA1 or BRCA2 mutation in women whose family histories are suggestive of inherited breast and/or ovarian cancer. It can also be used to estimate BC risk for each individual member of the family. BRCAPRO does not incorporate risk factors that are unrelated to family history.
The Tyrer–Cuzick (also called IBIS, see http://www.ems-trials.org/riskevaluator/) is a computer-based model that can be used to estimate the probability of carrying a BRCA1 or BRCA2 mutation as well as individual BC risk for the patient and for family members. In addition to factors related to family history, this model incorporates other well-established risk factors when calculating BC risk estimates.
The BOADICEA (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm) model (http://www.srl.cam.ac.uk/genepi/boadicea/boadicea_home.html), like the Tyrer-Cuzick, computes the risks of breast and ovarian cancer in women based on their family history. It is also used to calculate the probability that they are carriers of cancer-associated mutations in the BRCA1 or BRCA2 gene. This programme is free and is an example of translational research, where scientific software has been developed into a tool for healthcare professionals. In the United Kingdom, it is recommended as a risk assessment tool in the NICE clinical guidelines and has been incorporated in the guidelines of several countries for the management of familial BC.
2.3.4 Pathological Characteristics of Hereditary BC
Eighty per cent of invasive BCs arising in BRCA1 and BRCA2 carriers are invasive ductal carcinomas. A higher frequency of BRCA1 tumours is medullary carcinomas (9 % versus 2 % of sporadic BC), usually poorly differentiated and high grade but with a remarkably favourable prognosis, probably because of low incidence of lymph node metastasis. Notably, 11 % of medullary carcinomas carry BRCA1 germ-line mutations. By contrast, excess of invasive lobular and tubular carcinomas has been reported for BRCA2 relative to BRCA1 tumours [8].
BRCA1 tumours are more frequently high grade compared to sporadic tumours. They have a higher number of mitosis and show a high frequency of necrotic areas, a higher proportion of continuous pushing margins and a considerable lymphocytic infiltration. All these features point towards a more aggressive tumour type. Most BRCA2 tumours are grade 2/3 with high mitotic rates and have as a common feature continuous pushing margins.
Among sporadic tumours, 70 % are ER positive and 50 % are PR positive, and HER2 overexpression is observed in approximately 15 % of cases. In BRCA1 carriers expression of the hormone receptors is significantly lower from sporadic tumours, even when the ratio is adjusted for the younger age of the BRCA1 patients. A recent study examining pathology data from 4,325 BRCA1 and 2,568 BRCA2 mutation carriers reported that 78 % of tumours arising in BRCA1 carriers were ER negative, while only 23 % of tumours arising in BRCA2 mutation carriers were ER negative. Furthermore, HER2 overexpression was only observed in approximately 10 % of the tumours in mutation carriers. Consequently, 69 % of the BRCA1 tumours were triple negative (i.e. negative ER, PR and HER2; see ‘Molecular subtypes’, Sect. 13.2




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








