Naïve
Central memory
Effector memory
Resident memory
CD44
−/low
+
+
+
CD62La (L-selectin)
+
+
−
−
CCR7a
+
+
−
−
CD127 (IL-7Rα)
Varied
+
+
Varied
CD69
−
−
−
+
CD103 (integrin αE)
−
−
−
+
CD11a
Varied
Varied
Varied
+
Distribution
LN, Sp, PB
LN, Sp, PB, BM
Sp, PB, peripheral tissue
Peripheral tissue
Migration
Yes
Yes
Yes
No
5.4 Subsets of αβT Cells
The αβ T cells comprise multiple subsets (Fig. 5.1). Two important lineages of the αβT cells are CD4 T cells and CD8 T cells. These cells differ in how they recognize antigen and mediate effector functions. The CD4 T cell population consists of diverse helper T cells including Th1, Th2, Th17, Th22, Th9, Tfh, and Treg cells (immune suppressive T cells). Another subset of αβT cells is natural killer T (NKT) cells, which express invariant TCRs and share many functions with natural killer (NK) cells, a subset of innate type lymphocytes.
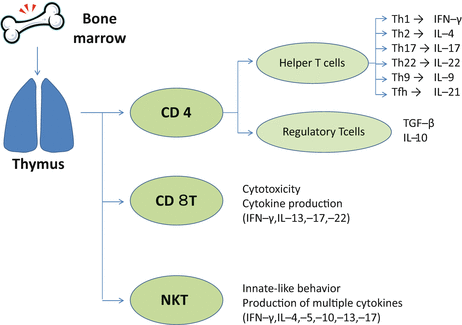

Fig. 5.1
Development of αβT cells. Bone-marrow–derived precursor cells enter the thymus to become thymocytes. Thymocytes complete their developmental steps to exit the thymus as CD4 T cells, CD8 T cells, or NKT cells expressing αβ T-cell receptors. The cytokine milieu induces differentiation of CD4 T cells into a variety of helper T (Th) cell-subsets such as Th1, Th2, Th17, Th22, Th9, and Tfh cells. CD4 T cells also differentiate to regulatory T (Treg) cells that downmodulate immune responses. Although CD8 T cells possess cytotoxicity, they can produce various cytokines depending on the inflammatory circumstances. Natural killer T (NKT) cells are a subset of αβT cells that share functions of T cells and NK cells. NKT cells behave like innate lymphocytes and promptly produce various cytokines on encounter with pathogens
5.5 CD4 T Cells
Host responses vary with infections. Whereas cutaneous exposure to Mycobacterium tuberculosis induces massive macrophage infiltration, helminthic infection promotes IgE production and activation of eosinophils. The discovery that CD4 T cells mediate these different responses led to the identification of heterogeneous CD4 T cell subsets (Table 5.2). The first two subsets identified were type 1 (Th1) and type 2 helper T (Th2) cells [89]. Later, IL-17 producing Th17 cells were identified to mediate some autoimmune diseases that had been attributed to Th1 cells. Currently, IL-9 producing Th9 cells, IL-22 producing Th22 cells, and germinal center-forming follicular helper T (Tfh) cells are recognized. Furthermore, CD4 T cells harbor immune-suppressive subsets such as Foxp3+ Treg cells, Tr-1, and Th3 cells. It is important to note, however, that each CD4 T cell can express cytokines or transcription factors that are not specific for its subset. CD8 T cells can produce a series of cytokines that are classified into the CD4 T cell subsets. Such CD8 T cells are called Tc1, Tc2, Tc17, or Tc22. Therefore, it seems that each T cell possesses functional plasticity or flexibility. The question whether the CD4 or CD8 T cell subsets represent a terminally differentiated state or they just reflect certain aspects of their transient states remains to be solved.
Table 5.2
Subsets of CD4 T Cells
Inducing cytokines | Master regulator | Effector cytokines | Inhibitors | Function | Host defense | Pathology | |
|---|---|---|---|---|---|---|---|
Th1 | IFN-γ | T-bet | INF-γ | IL-4 | Activation of macrophages, Ig class switch to IgG1/G3 (human) or IgG2a/G3 (mouse) | Intracellular pathogens | Granulomatous diseases, inflammatory bowel disease |
IL-12 | |||||||
Th2 | IL4 | GATA3 | IL-4 | IFN-γ | Activation of mast cells, basophils, eosinophils, alternative macrophages, barrier function, class switching to IgE | Helminths | Atopic dermatitis, allergic asthma |
IL-5 | |||||||
IL-10 | |||||||
IL-13 | |||||||
Th17(β) | IL-6 | RORγt | IL-17A/F | IL-2 | Recruitment of neutrophils | Extracellular bacteria and fungi | Undefined |
TGF-β | IL-10 | IFN-γ | |||||
IL-4 | |||||||
Foxp3 | |||||||
T-bet | |||||||
Th17(23) | IL-6 | RORγt | IL-17A/F | Ditto | Ditto | Ditto | Psoriasis |
IL-23 | T-bet | IL-22 | TGF-β1 | RA | |||
IL-1β | IFN-γ | MS | |||||
TGF-β3 | GM-CSF | ||||||
Th22 | IL-6 | AhR (?) | IL-22 | TGF-β1 | Induction of defensins | Klebsiella pneumoniae | Psoriasis |
TNF-α | RORγt (?) | ||||||
Notch (?) | |||||||
Th9 | IL-4 | PU.1 | IL-9 | Undefined | Production of mucus | Undefined | Undefined |
TGF-β | IRF-4 | ||||||
Tfh | IL-6 | Bcl6 | IL-21 | IL-2 | B cell maturation, Ig class switching, memory B cell development | B-cell–mediated protection | Undefined |
Blimp1 | |||||||
Foxp3+ Treg | IL-2 | Foxp3 | TGF-β | IL-6 | Maintenance of peripheral tolerance, attenuation of immune response | Tuning of inflammation | Impaired host defense, cancer |
TGF-β | IL-10 | RORγt | |||||
IL-35 | HIF1a | ||||||
Tr1 | IL-27 | c-Maf | IL-10 | Undefined | Ditto | Ditto | Undefined |
TGF-β | |||||||
Th3 | Undefined | Undefined | TGF-β | Undefined | Ditto | Ditto | Undefined |
LAG3 Treg | Undefined | Undefined | IL-10 | Undefined | Ditto | Ditto | Undefined |
5.5.1 Th1 Cells
(Summary) Th1 cells produce IFN-γ and protect against intracellular pathogens such as Mycobacteria, Listeria, Toxoplasma, and viruses. Th1 cells dominate during the early phase of contact dermatitis.
5.5.1.1 Characteristics of Th1 Cells
Th1 cells are defined by secretion of IFN-γ. Th1 cells express IL-12 receptor β1 and β2, IL-18 receptor, E-selectin, P-selectin, CXCR3, and CCR5. IFN-γ activates macrophages, dendritic cells, CD8 T cells, NK cells, and B cell class-switching to IgG1 and IgG3 in humans (IgG2a and IgG3 in mice). Th1 cells suit the immune response to the protection against intracellular pathogens, such as Mycobacteria, Listeria, Toxoplasma, and viruses.
5.5.1.2 Differentiation of Th1 Cells
IFN-γ promotes differentiation of Th1 cells in synergy with IL-12, IL-18 and type I IFN (IFN-α/β) (Fig. 5.2). IL-12 activates the signal transducer and activator of transcription (STAT) 4, a modifier for over 4,000 genes encoding factors related to Th1 cells, such as IFN-γ and receptors for IL-12 and IL-18 [150]. IFN-γ activates STAT1, which reinforces differentiation to Th1 cells. Activated STAT4 and STAT1 induce expression of T-bet (the master regulator for Th1 cells) encoded by TBX21, which regulates genes such as Ifng that affect Th1 cell-commitment [75, 83, 139]. Th1 cells can differentiate without STAT4 and T-bet via Hlx, Runx3, and Ets. It is not fully understood how such alternatively differentiated Th1 cells function.
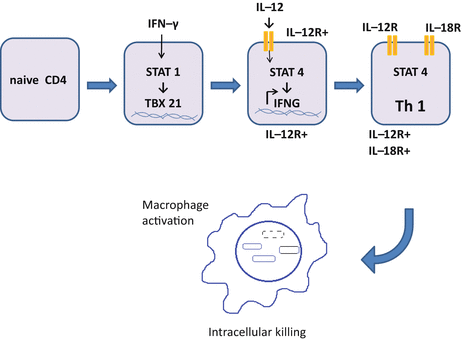

Fig. 5.2
Differentiation of Th1 cells. Binding of IFN-γ to its receptor on naive CD4 T cells induces the expression of TBX21 encoding T-bet, in a STAT1-dependent manner. T-bet induces the expression of IL-12Rβ2, which combines with IL-12Rβ1 (expressed on naive CD4 T cells) to form the receptor for IL-12 (IL-12R). In concert with T-bet, binding of IL-12 activates STAT4 and induces the expression of IFNG gene encoding IFN-γ, by which the receptor for IL-18 (IL-18R) is expressed. Fully committed Th1 cells produce a large amount of IFN-γ and activate macrophages
5.5.2 Th2 Cells
(Summary) The signature cytokine of Th2 cells is IL-4. Th2 cells promote humoral immune response, contribute to host defense against extracellular pathogens such as parasitic worms, and play a role in atopic diseases. Epithelial cell-derived cytokines (IL-25, IL-33, and TSLP) initiate Th2 cell-differentiation.
5.5.2.1 Characteristics of Th2 Cells
Th2 cells are defined by secretion of IL-4, IL-5, and IL-13. IL-24, IL-25 (IL-17E), and IL-31 are produced by Th2 cells as well. Receptors for IL-25, IL-33 (an IL-1 family alarmin), TSLP (thymic stromal lymphopoietin), CRTH2 (chemoattractant receptor-homologous molecule on Th2 cells), and chemokine receptors such as CCR3, CCR4, and CCR8 are expressed on Th2 cells. IL-4, IL-5, and IL-13 help B cells to produce immunoglobulin (Ig). IL-4 initiates Th2 cell differentiation and promotes Ig class-switching to IgG4 and IgE in humans (IgG1 and IgE in mice). IL-4 and IL-13 induce alternatively activated macrophages while suppressing IFN-γ-induced classically activated macrophages. IL-4, IL-5, and IL-13 mobilize eosinophils, basophils, and mast cells, and increase mucous secretion. IL-31 (an IL-6 family cytokine) promotes dermatitis in mice [34]. Eosinophils and Th2 cells express CRTH2, which is a receptor for prostaglandin (PG) D2 and induces chemotaxis of these cells to the inflammatory sites. PGD2 is produced by mast cells, which reside in epithelial tissues including the skin. Th2 cells cause allergic responses including AD, pollen rhinitis, and asthma.
5.5.2.2 Differentiation of Th2 Cells
In contrast to other Th subsets, activation of antigen-presenting cells (APCs) is not sufficient to provide IL-4. Instead three Th2-promoting cytokines (IL-25 , IL-33 , and TSLP ) are necessary for Th2 cell differentiation (Fig. 5.3). Epithelial cells produce these cytokines by antigenic or environmental stimuli. IL-25 and IL-33 recruit and activate innate immune cells such as basophils, eosinophils, mast cells, NK cells, and recently identified natural helper (NH) cells. These innate cells produce IL-4 in addition to IL-5 and IL-13. TSLP is an IL-7-like cytokine that activates B cells and DCs. TLSP primes DCs to express CD40 ligand (CD40L) and to decrease the production of p40 subunit of IL-12 [100]. CD40L thus induced on DCs triggers naive CD4 T cells to produce IL-4, IL-5, and IL-13 [64]. IL-4 activates STAT6 followed by expression of GATA3, the master regulator of Th2 cells encoded by Gata3. GATA3 transactivates genes such as Il4, Il5, Il13, and Gata3, all of which reinforce commitment to Th2 cells [149].
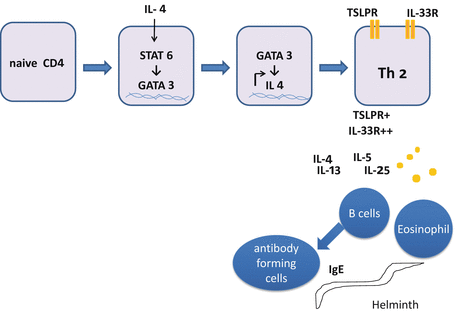

Fig. 5.3
Differentiation of Th2 cells. Binding of IL-4 to its receptor on naive CD4 T cells suppresses the expression of IL-12Rβ1 and induces expression of GATA3 encoding GATA3, in a STAT6-dependent manner. GATA3 transactivates a set of genes required for Th2 cell differentiation. Fully committed Th2 cells express receptors for TSLP (TSLPR) and IL-33 (IL-33R). IL-4, IL-5, IL-13, and IL-25 produced by Th2 cells activate basophils and B cells. B cells further differentiate to antibody forming cells (AFCs, or plasma cells) to produce immunoglobulins such as IgE to protect the body against helminth infection
5.5.2.3 Th2 Cells and Skin Pathology
Th2 cells are engaged in the allergic skin diseases such as AD. AD is a chronic and relapsing inflammatory skin disease with severe pruritus. AD presents early in life, in general, and is associated with sensitivity to food or inhalant allergens, indicating a complex interaction between the skin and systemic immune system. Dry skin is a feature of AD, which suggests breakage of the skin barrier with these patients. Indeed, certain AD patients harbor mutations in the FLG gene, which encodes filaggrin (filament-aggregating protein), a key protein in facilitating epidermal differentiation and the skin barrier [102, 131, 151]. Breakage of the skin barrier opens the entry sites for exogenous antigens leading to stimulation of keratinocytes that produce Th2-promoting cytokines, i.e., IL-25, IL-33, and TSLP, which stimulate group 2 innate lymphoid (ILC2) cells including NH cells to produce IL-5 and IL-13 [134]. TSLP stimulates DCs to express CD40L and reduce IL-12 p40, thereby skewing activated T cells toward Th2 cell-differentiation. TSLP-primed DCs secrete CCL17 (TARC) and CCL22 (MDC), both of which attract Th2 cells to the site of inflammation [109, 133]. Therefore, barrier dysfunction and production of IL-25, IL-33, and TSLP play a central role in the exacerbation of Th2 cell-mediated inflammation.
Advancing biologics provide clues to understand Th2 cell-mediated immunity in skin diseases although evidence is conflicting now as follows [42]. Anti-IL-5 mepolizumab did not ameliorate AD whereas the number of blood eosinophils decreased. Efficacy of anti-IgE omalizumab onto AD differs among the studies, reflecting heterogeneity of the disease. Clinical studies are underway for anti-IL-31 antibody, CRTH2 inhibitor, TSLP receptor binding protein, and IL-4Rα binding protein antagonizing IL-4 and IL-13. These studies should clarify the role of Th2 cells in skin diseases.
5.5.3 Th17 Cells
(Summary) IL-17 production defines Th17 cells that are divided in Th17(β) or Th17(23) , which are induced by the combination of IL-6 and TGF-β or of IL-6, IL-23, and IL-1β, respectively. Th17 cells are supposed to cause autoimmune diseases such as psoriasis, rheumatoid arthritis, and multiple sclerosis. IL-23 is critical for activation of Th17 cells.
5.5.3.1 Characterization of Th17 Cells
Self-reactive Th1 cells had been postulated to cause autoimmune diseases [70]. Indeed, loss of T-bet prevented experimental autoimmune encephalomyelitis (EAE), a mouse model for multiple sclerosis in humans [12]. However, EAE can occur in the absence of IFN-γ or its receptor [44, 153]. Furthermore, studies of gene-targeted mice for IL-12 (a p70 heterodimer composed of a p35 subunit and a common p40 subunit shared by other cytokines) came to a seemingly contradictory conclusion: p40−/− mice were resistant to EAE, whereas p35−/− showed even more susceptibility than wild-type. This finding indicated that a molecule other than IL-12 (p35/p40)—identified later as IL-23 (p19/p40) that activates Th17 cells—fulfills the function previously attributed to IL-12 [9, 55]. Such difficulties in explaining the autoimmune diseases simply on the basis of the Th1/Th2 paradigm led to the identification of CD4 T cells that produce IL-17, termed Th17 cells [29, 53, 73, 148]. Signature cytokines of Th17 cells are IL-17A and IL-17F, both of which recruit and activate neutrophils. Th17 cells express CCR6 and its ligand CCL20 [2], and produce IL21 and IL-22 as well. Th17 cell subset seems to include at least two subsets; Th17(β) and Th17(23) [71].
The natural role of Th17 cells is protection against bacterial and fungal species. IL-17 recruits and generates neutrophils through the production of chemokines and GM-CSF (granulocyte macrophage colony stimulating factor), respectively. Recruited neutrophils then besiege extracellular bacteria and fungi. Defense by Th17 cells is oriented to mucocutaneous surface rather than to the whole body, and its main targets are Staphylococcus aureus, Klebsiella pneumoniae, and Candida albicans [6, 26, 68]. Autosomal dominant hyper IgE syndrome (HIES or “Job’s syndrome”) is explained by dysfunctional Th17 cells due to the mutation in STAT3 that is activated by IL-6 and IL-23. These patients are susceptible to candidiasis and staphylococcal infections [84]. This impairment is due to lack of IL-17, as autosomal recessive IL-17RA deficiency or autosomal dominant IL-17F deficiency leads to chronic mucocutaneous candidiasis (CMC) with Staphylococcus aureus dermatitis as well [106]. Likewise, autoantibodies against IL-17A, IL-17F, and IL-22 cause CMC [67, 107].
5.5.3.2 Differentiation and Activation of Th17 Cells
Differentiation of Th17 cells requires STAT3 and RORγt , both of which are regulated by key cytokines including IL-1β, IL-6, IL-21, IL-23, and transforming growth factor-beta1 (TGF-β1 or TGF-β otherwise indicated; Fig. 5.4). Combinations of these cytokines induce two subsets in Th17 cells, namely Th17(β) and Th17(23), and another relative Th22 (described later) [71]. Th17(β) cells express IL-17A, IL-17F, IL-10, CCL20, and CXCR6, and are differentiated by the combination of IL-6 and TGF-β. On the other hand, Th17(23) cells, which are more pathogenic and express IL-22, CCL9, and CXCR3 in addition to IL-17A and IL-17F, are differentiated by the combination of IL-1β, IL-6, and IL-23 [52]. TGF-β1 rather inhibits IL-22 expression of Th17 cells. IL-21 and IL-1β expand and promote Th17(β) and Th17(23) in vitro [56, 69, 125, 138, 159].
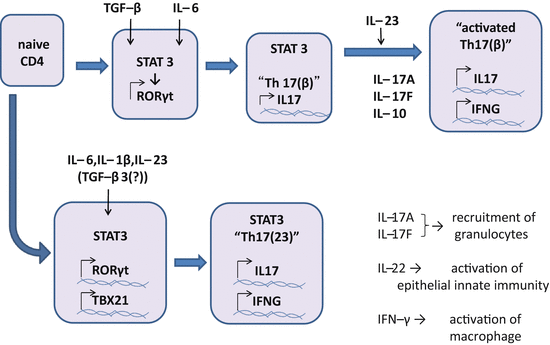

Fig. 5.4
Differentiation of Th17 cells. Recent studies revealed there are two pathways in differentiation of Th17 cells. In one pathway, combination of IL-6 and TGF-β induces RORC encoding RORγt in a STAT3-dependent manner. RORγt commits the cell to Th17(β), a Th17 lineage that produces IL-17A, IL-17 F, and IL-10. Th17(β) can be activated by IL-23 and produce IL-22, IFN-γ, and GM-CSF. GM-CSF is suggested to play a role in the inflammation of the central nervous system. In another pathway, combination of IL-6, IL-1β, and IL-23 induces both RORC and TBX21 in a STAT3-dependent manner, by which Th17(23) are differentiated. Th17(23) cells produce IL-17A, IL-17 F, IL-22, and IFN-γ. IL-17A and IL-17 F recruit granulocytes. IL-22 activates epithelial innate immunity to reinforce the protective function of skin and mucosa. IFN-γ activates macrophages
The most important cytokines that regulate Th17 cell-differentiation are IL-6 and IL-23. They activate STAT3 to regulate genes such as Il17a/f, Il23r, Il21, and Rorc. Rorc encodes RORγt (also referred as RORc), which is the master regulator for Th17 cells. TGF-β is a pleiotropic cytokine produced by various kinds of cells. It works as an anti-inflammatory cytokine and induces Foxp3+ Treg cells. However, in the presence of IL-6, TGF-β induces RORγt [63, 167] in a fashion other than the Smad2 and Smad3 pathway [141].
RORγt is a T-cell–specific splice variant of RORγ, which is an orphan retinoid receptor expressed in various tissues and engaged in development of thymus, lymphoid tissue inducer cells, and IL-22-producing subset of NK cells as well [80, 120, 121, 140]. RORα, another member of the orphan retinoid receptor family, also induces Th17 cell-differentiation with RORγt [159]. Digoxin and its derivatives inhibit RORγt and suppress Th17 cell-differentiation [62].
With respect to Th17(23) cells, it is not fully understood how IL-6, IL-23, and IL-1β induce RORγt without TGF-β1. It is reported that a combination of TGF-β3 and IL-6 induces very pathogenic Th17 cells—distinct from Th17(β) cells—in mouse EAE, and that IL-23 is required for the production of TGF-β3 by the developing Th17 cells [78]. It is noteworthy that Th17(β) cells are not pathogenic unless they are exposed to IL-23, whereas Th17 cells induced by TGF-β3 and IL-6 are very pathogenic without IL-23. Whether the TGF-β3-induced Th17 cells are equivalent to Th17(23) is not confirmed yet. At least it is clear that IL-23 plays a central role in the generation of pathogenic Th17 cells.
Other transcription factors such as aryl hydrocarbon receptor (AhR), Batf, IκBζ, IRF-4, and Runx1 are known to promote Th17 differentiation, although the precise mechanism is ambiguous [71].
5.5.3.3 Metabolism and Other Factors Affecting Differentiation of Th17 Cells
Metabolic state affects development of Th17 cells. Indole-amine-pyrrole 2,3-dioxygenase (IDO) is an enzyme that catabolizes tryptophan and suppresses the activity of mTORC1 (the metabolic target of rapamycin complex 1) in T cells [74]. Lack of IDO increases production of IL-17 and Th17 cell infiltration [27]. Halofuginone inhibits Th17 cell differentiation through amino acid starvation response, which is correlated with mTOR (the metabolic target of rapamycin) [137]. Thus, mTOR promotes differentiation of Th17 cells. Hypoxia-inducible factor (HIF)-1 forms a complex with RORγt and recruits acetyl transferase p300 to the Il17 locus [30, 126]. HIF-1α operates on a metabolic state of the cell and switches the oxidative phosphorylation state to an aerobic glycolysis state during the hypoxia [123]. Therefore, the hypoxic condition supports Th17 cell differentiation. Notably, the above regulations affect differentiation of memory T cells and Foxp3+ Treg cells in an opposite way. It remains unclear whether such mTOR-mediated metabolism affects Th17-mediated skin diseases such as psoriasis vulgaris.
Environmental pollutants affect Th17 cell differentiation through the aryl hydrocarbon receptor (AhR) [108, 144]. AhR is expressed selectively in Th17 cells; and its ligation accelerates onset of EAE in mice in a Th22 cell-dependent manner [144]. The fate of T cells depends on the ligands for AhR. For example, 2,3,7,8-tetrachlorodibenzo-p-dioxin activates AhR and induces Foxp3+ Treg cells, ameliorating EAE, whereas 6-formylindol[3,2-b]carbazole interferes with Treg cell-development and boosts Th17 cell differentiation, exacerbating EAE [108]. Thus, various aromatic molecules may regulate Th17 and Treg cell differentiation depending on the context. It is under investigation how AhR affects skin pathology.
5.5.3.4 Th17 (and Th22) Cells in Skin Diseases
Th17 cells (and Th22 cells ) have been studied in the context of endodermal barriers such as intestinal and respiratory tracts. However, these cells occupy important roles in the skin as well [147]. Irritated keratinocytes produce IL-1β and IL-6 that stimulate LCs and dermal DCs to produce IL-23 and migrate to regional lymph nodes, where Th17 and Th22 cells are differentiated in response to antigens presented by these APCs. Skin-homing Th17 and Th22 cells produce IL-17A, IL-17F, IL-22, and TNF-α. These ligands affect keratinocytes to produce various cytokines (IL-32 and IL-36), chemokines (CXCL1 and CCL20), and antimicrobial peptides (HBD-2 and S100A7/proriasin, and S100A15/koebnerisin); all of which enhance local inflammatory responses.
Psoriasis is a prototype of dysregulated Th17 cell-mediated immune response in the skin. Psoriasis was thought to be a Th1/Tc1 autoimmune disease because IFN-γ-producing Th1 and Tc1 (CD8 T cells) cells were identified from the lesion. Ustekinumab , an anti-IL-12p40 monoclonal antibody, was originally chosen for this reason. However, it is now appreciated that neutralization of IL-12p40—a shared subunit between IL-12 and IL-23—rather targets the IL-23/IL-17 axis and Th17 cell-mediated pathway. Genomewide association study (GWAS) reinforced the importance of the IL-23 signaling pathway through identification of IL23R, IL23A, and IL12B loci as psoriasis-susceptibility genes [17, 93]. Furthermore, an antihuman IL-17A monoclonal antibody was effective for psoriasis in a clinical study [61]. Thus, the onset of psoriasis may arise from dysregulated IL-17-dependent expansion of CD4 (Th17) and CD8 (Tc17) cells. Although there is no clear explanation for the role of IFN-γ-producing (Th1 and Tc1) cells in the psoriatic lesion, these cells may be derived through developmental plasticity between Th17 and Th1 cells. Another unsolved question is the role of TNF-α. As inhibition of TNF suppresses IL-17-pathway genes in responders, at least there is a link between these cytokines [160].
5.5.4 Th22 Cells
(Summary) Th22 cells produce IL-22 and TNF-α, but not IL-17. The role of IL-22 is to activate the innate immune response of the epithelium. Th22 cells mediate acanthosis and skin lesions of psoriasis vulgaris and AD. IL-22 producing CD8 T cells are called Tc22.
5.5.4.1 Function of IL-22
The IL-10 family cytokine IL-22 operates in both pro- and anti-inflammatory ways [115, 161]. Th1, Th17, Th22, and γδT cells produce IL-22 in humans [132]. Interaction with LCs via CD1a enhances IL-22 production in self-reactive T cells [32]. IL-22 activates epithelial innate responses, which can be protective or detrimental [43]. IL-22 suppresses inflammatory bowel disease and acute liver inflammation in mice whereas it mediates mucosal host defense against gram-negative bacterial pneumonia [6]. An example of detrimental effect is epithelial hyperplasia in psoriasis, which can be induced by IL-22 in human artificial skin cultures [13]. In mice, IL-22 produced by Th17 cells mediates IL-23-induced dermal inflammation and acanthosis [164]. Increased IL-22 production is correlated with the squamous cell carcinoma occurring in immunocompromised transplant recipients [163]. Therefore, IL-22 and Th22 cells are involved in various skin diseases such as psoriasis vulgaris, AD, contact dermatitis, scleroderma, and tumorigenesis [48, 163].
5.5.4.2 Characterization of Th22 Cells
Certain helper T cells, CCR10+ ones in particular, are called Th22 cells as they produce IL-22 but not IL-17 [38, 43, 116, 143, 164, 166]. Th22 cells mediate dermal inflammation and acanthosis in an IL-23-dependent manner [164]. Skin-homing memory CD4 T cells expressing CCR4, CCR6, and CCR10 are supposed to be Th22 cells (or T-22 cells when Tc22 cells are also considered) [38, 43, 95, 143]. Th22 cells may regulate epidermal immunity, remodeling of epidermis, dermal inflammation, and acanthosis.
5.5.4.3 Differentiation of Th22 Cells
Plasmacytoid DCs induce Th22 cells from naive T cells in a way dependent on both IL-6 and TNF [38] (Fig. 5.5). Specific transcription factor is not defined for Th22 cell differentiation. AhR and RORγt are at least important for IL-22 production [144, 145]. Notch induces IL-22 secretion in CD4 T cells by stimulating AhR [4]. TGF-β inhibits IL-22 secretion through expression of c-Maf [116]. In a transcriptome analysis of human Th22 cell clones, the presence of BNC-2 and FOXO4 expression was observed whereas RORC2, GATA3, and T-bet were reduced [43]. It remains unclear whether these transcription factors play roles in Th22 cell-differentiation.
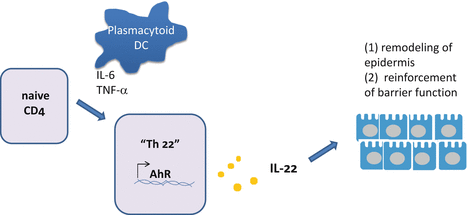

Fig. 5.5
Differentiation of Th22 cells. Th22 cells can be induced from naive CD4 T cells by plasmacytoid dendritic cells (pDCs) in a manner dependent on IL-6 and TNF-α. Aryl hydrocarbon receptor (AhR) encoded by the AhR gene is one of the candidates that regulate the differentiation of Th22 cells. IL-22 produced by Th22 cells (or by Tc22 cells, i.e., CD8 T cells producing IL-22) induces remodeling of the epidermis and mucous epithelium to reinforce the protective function of the surface barrier
5.5.4.4 Th22 Cells in Skin Diseases
Accumulation of Th22 and Tc22 cells indicate involvement of these cells in psoriasis and AD [43, 95, 156]. The majority of Th22 cells respond to CD1a [32]. Therefore, Th22 and Tc22 cells may proliferate in response to lipid antigens presented by the LC network in the epidermis and mediate acanthosis of AD. On the other hand, a dioxin-intoxicated patient showed an increased number of dermal Th22 cells suggesting a pathogenic role, however, how IL-22 contributes to the dioxin-induced skin lesions—chloracne—remains unresolved [14].
5.5.5 Th9 Cells
(Summary) Th9 cells produce IL-9, which promotes proliferation of mast cells and plays a role in allergy. Th9 cells are induced by IL-4 in the presence of TGF-β.
5.5.5.1 Function of IL-9
A subunit of the IL-9 receptor is the common cytokine receptor γ (cγ) chain, which is shared with the receptors for cytokines including IL-2, IL-4, IL-7, IL-13, IL-15, IL-21, and TSLP. IL-9 promotes proliferation of erythroid progenitor cells, B cells, B1 B cells, mast cells, and fetal thymocytes in mice. IL-9 is highly expressed in the lung of patients with asthma [41]. Neutralization of IL-9 reduces production of IL-5 and IL-13, indicating a link between IL-9 and Th2 type immune response [154]. Th17 and Treg cells also produce IL-9 [40].
5.5.5.2 Differentiation of Th9 Cells
IL-4 and TGF-β induce Th9 cells in a pathway dependent on interferon regulatory factor (IRF) 4 [135] (Fig. 5.6). IL-4 inhibits differentiation of TGF-β-induced Foxp3+ Treg cells and induces IL-9+IL-10+Foxp3- T cells instead [31]. Alternatively Th9 cells are induced from Th2 cells [145]. The transcription factor PU.1 is required for Th9 cell differentiation [18].
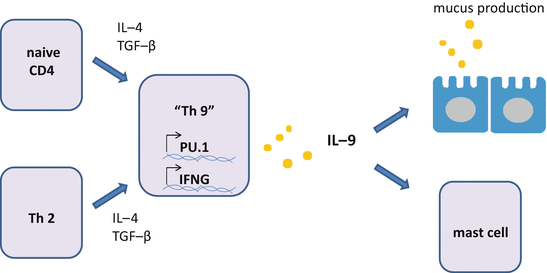

Fig. 5.6
Differentiation of Th9 cells. Combination of IL-4 and TGF-β induces Th9 cells from naive CD4 T cells or Th2 cells. It is claimed that differentiation of Th9 cells requires the transcription factor PU.1. IL-9 produced by Th9 cells activates mast cells and enhances the production of mucus
5.5.5.3 Role of Th9 Cells
Although IL-9 seems to exacerbate allergy [18], unclear issues remain. Blockade of IL-9 or IL-9Rα ameliorates EAE; whereas disruption of IL-9Rα gene worsens it [94]. Human IL9 is located in the region encoding IL3, IL4, IL5, IL13, and GMCSF (5q31–5q35), whereas mouse Il9 is on chromosome 13, not on chromosome 11 where Il3, Il4, Il5, Il13, and Gmcsf are clustered. This indicates the function of IL-9 in humans should not be extrapolated from the knowledge from mice.
5.5.6 Follicular Helper T (Tfh) Cells
(Summary) Tfh cells help B cells, supporting germinal center formation, affinity maturation, and generation of long-lived memory B cells.
Tfh cells reside in lymph nodes and help B cells [28]. Tfh cells produce IL-21, which promotes germinal center formation and generation of plasma cells. Surface markers for Tfh cells are PD-1, CXCR5, BTLA, PSGL-1, and ICOS. Tfh cells express BCL6, a transcription factor induced by IL-6 and IL-21. The role of Tfh cells in the skin is not known.
5.5.7 Regulatory T (Treg) Cells
(Summary) Gershon and Kondo showed that thymus-derived immune cells suppress antibody formation in an antigen-specific manner [51]. Through the pursuit of the suppressor T cells, several subsets were claimed to mediate suppression. Foxp3+CD4+ regulatory T (Treg) cells (Treg cells otherwise indicated) constitute the most characterized subset.
5.5.7.1 Foxp3+ Treg Cells
(Summary) Foxp3+ Treg cells maintain peripheral tolerance, preventing autoimmune diseases and limiting immune pathology, although they may deteriorate antitumor immunity and chronic infections [146]. The transcription factor Foxp3 is critical for Treg cell development in mice and humans [46, 60, 66]. Foxp3+ Treg cells contain thymus-derived (tTreg) and peripherally induced (pTreg) cells.
5.5.7.2 Characterization of Foxp3+ Treg Cells
Neonatally thymectomized (ntx) mice before day 3 of age spontaneously develop organ-specific autoimmune diseases later in life, such as gastritis, thyroiditis, and oophoritis. Alternatively, fractionated irradiation on the thymus induces similar autoimmune diseases in rats. These experiments indicated that thymus generates distinctive T cells that suppress immune responses. Through screenings of T cell subsets on the basis of surface markers, CD25+CD4+ T cells were shown to protect ntx mice from the autoimmune diseases [117]. Discovery of Foxp3, selectively expressed by the majority of CD25+CD4+ T cells, has enabled us to investigate the regulatory/suppressor T cells on a molecular basis.
Currently, Foxp3+CD4+ Treg cells are recognized as the major T cells with suppressive activity [114]. Expression of Foxp3 does not always correlate with suppression, particularly in humans [85]. Nonetheless, absence of Foxp3 results in a fatal autoimmunity in mice and humans, i.e., Scurfy phenotype and IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome , respectively [76]. IPEX syndrome is a fatal disease characterized by autoimmune enteropathy, psoriasiform dermatitis, eczematous dermatitis, cheilitis, nail dystrophy, and autoimmune endocrinopathies (early onset type I diabetes mellitus and thyroiditis) [57, 76]. The hypomorphic version of IPEX syndrome is often accompanied by serum hyper-IgE, eosinophilia, alopecia areata , chronic urticaria secondary to food allergies, and bullous pemphigoid , indicating engagement of Treg cells in these conditions [57].
Treg cells represent about 10 % of the peripheral CD4+ T cell subset. Most of them express CD25 (IL-2Rα), cytotoxic T lymphocyte-associated antigen 4 (CTLA4 , a ligand for CD80/CD86), and glucocorticoid-induced TNF receptor (GITR) . Although these markers indicate an activated state, Treg cells are anergic, nonproliferative to antigen stimulation, and hardly produce cytokines except anti-inflammatory IL-10 and TGF-β .
5.5.7.3 Subsets of Treg Cells
Treg cells that arise in the thymus are called “thymus derived Treg (tTreg) cells .” CD4+ T cells acquiring Foxp3 expression in the periphery are called “peripherally derived Treg (pTreg) cells ” [1] (Fig. 5.7).
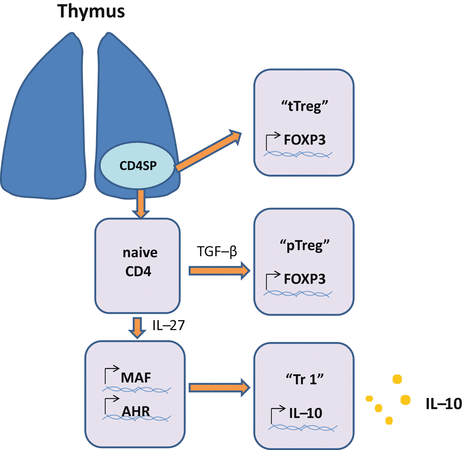

Fig. 5.7
Differentiation of Treg cells. Regulatory T (Treg) cells consist of Foxp3+ and Foxp3- Treg cells. Foxp3+ Tregs cells are further divided into thymically derived Treg (tTreg) cells and peripherally induced (pTreg) cells. Development of tTreg cells occurs in the thymus. Differentiation of pTreg cells occurs in the periphery and depends on TGF-β. Continuous exposure to antigen at a low dose seems to promote differentiation of pTreg cells. Tr1 cells are induced by antigen stimulation in the presence of IL-10, vitamin D3, type I interferon, IL-27, and ICOS in a manner dependent on c-Maf and AhR (aryl hydrocarbon receptor). Th3 cells, which are induced in oral tolerance models in animals, are suggested to be equivalent to pTreg cells. Differentiation of LAG3 Treg cells is obscure
It is a fundamental question whether tTreg and pTreg cells are discernible. Helios , a member of the Ikaros family of transcription factors, is expressed at a high level in tTreg cells [142]. However, intravenously injected peptide can induce expression of Helios in pTreg cells [54]. Thus, Helios may be an activation marker rather than a differential for subsets [3]. An epigenetic approach identified a difference in degree of demethylation of the Foxp3 locus between tTreg and pTreg cells: the Foxp3 locus is fully demethylated in tTreg cells but not in pTreg. It is speculated that unstable expression of Foxp3 in pTreg cells associates with incomplete demethylation in Foxp3 [45]. However, it is technically impossible to discriminate the methylation state in living cells. To date, no biomarkers are identified for tTreg and pTreg cells. However, it is critical to determine the contribution of these subsets in inflammatory diseases or tumor immunity before we develop Treg cell mediated immune therapy.
5.5.7.4 Differentiation of tTreg Cells
TCR signaling is required for Foxp3 expression and lineage commitment to tTreg cells in developing CD4+CD8+ double positive (DP) thymocytes [65]. Expression of CD25, CD5, and CTLA4 is the first indication of commitment to tTreg cells. CD5 attenuates TCR signal strength by recruiting tyrosine phosphatase SHP-1. TCR specificity is decisive for tTreg cell differentiation. Myelin basic protein-specific TCR transgenic mice on a Rag-deficient state succumb to fatal EAE because tTreg cells cannot develop with the transgenic TCR [72, 99]. Thymic selection of tTreg cells is instructed by TCRs recognizing self peptide–MHC ligands; and signals by IL-2, IL-7, and IL-15 are required for development [65]. TGF-β signaling is not required for commitment of tTreg cells, although it promotes survival of tTreg precursors [101].
5.5.7.5 Differentiation of pTreg Cells
TCR signaling and TGF-β induce expression of Foxp3 in CD4+ T cells in vitro and in vivo. Retinoic acid (RA) enhances the induction of Foxp3 in this context [90]. A high level of RA (1 nM) maintains the TGF-β-mediated pTreg cell-differentiation by abrogating effects of IL-6 and nitric oxide, which promotes differentiation of Th17 and Th1 cells, respectively, in vitro [77]. Retinoids are functional analogues of RA. They are used topically or systemically to treat acne vulgaris, photoaging, psoriasis, pityriasis rubra pilaris, cutaneous T cell lymphoma, and eczema. It remains obscure to what extent retinoids exert their effects through the modulation of pTreg cells.
5.5.7.6 Treg Cells in the Skin
Human skin contains a resident population of Treg cells representing 5–10% of the total skin T cells [23, 24]. Treg cells are activated during skin inflammation and attenuate subsequent autoimmune reactions. Antigen-experienced Treg cells are maintained in the same tissue after the resolution of inflammation, and are promptly activated on antigen re-exposures. Therefore Treg cells confer “regulatory memory” to the skin [113]. LCs can activate skin resident Treg cells in the absence of exogenous antigen in vitro. However, LCs can activate Tem cells to produce IFN-γ and IL-17 in the presence of pathogenic antigens such as Candida albicans [124]. These mechanisms may operate immune homeostasis in the skin.
5.5.7.7 Mechanism of Suppression
Treg cells have multiple modes of suppression that can be divided in three classes [118]: (a) humoral factor-mediated suppression, (b) cell-contact–dependent suppression, and (c) functional modification of APCs. Treg cells produce immunosuppressive cytokines such as TGF-β , IL-10 , and IL-35 (heterodimer of EBI3 and p35 subunit of IL-12). Absorption of IL-2 (one of the T-cell growth factors) by CD25 (IL-2 receptor α) induces apoptosis of T cells nearby. Treg cells can induce apoptosis of neighboring T cells and APCs through cell-to-cell contact by granzyme – or perforin -dependent manner. CD39 and CD73 expressed by Treg cells generate peri-cellular adenosine, which is a strong immune suppressor. Moreover, Treg cells transmit cyclic AMP (cAMP) into the target T cell through a gap junction, by which proliferation and IL-2 production of the target are inhibited. Treg cells constitutively express CTLA4 , which downmodulates CD80/CD86 on APC and induces indoleamine 2,3-dioxygenase (IDO) , which catabolizes essential amino acids tryptophan to kynurenines that are toxic to T cells. LAG3 expressed by a subset of Treg cells mimics CD4 and binds to MHC-II, thereby attenuating the efficiency of antigen presentation by APCs.
Mechanism of suppression is more complicated in vivo. Treg cells coordinate their phenotype to match the type of immune response to be suppressed by sharing transcriptional regulation. A subtype of Treg cells suppresses Th1 cells, Th2 cells, or Th17 cells in a way dependent on T-bet, IRF4, or Stat3, respectively [15]. Furthermore, proper distribution of Treg cells in nonlymphoid tissues is important. For example, CCR4 expression is necessary for Treg cells to distribute in the skin to maintain the immune tolerance there [119].
Stability of a suppressive phenotype of Treg cells is another factor that affects the peripheral tolerance. Semaphorin-4a expressed on APCs ligates neuropilin-1 (Nrp1) on Treg cells to increase the nuclear fraction of Foxo3a resulting in stability of Treg cells [33].
5.5.7.8 Evidence for Presence of Treg Cells That Do Not Express Foxp3
(Summary) Tr1, Th3, and CD4+CD25-LAG3+ Treg cells are known to possess a suppressive function. These T cells do not express Foxp3.
Maintenance of T cell-tolerance depends on both central (thymic) and peripheral (nonthymic) tolerance. Absence of Foxp3+ Treg cells results in severe inflammation of multiple organs (such as lungs, liver, and skin). However, an additional defect in the central tolerance by the loss of Aire (autoimmune regulator gene) does not extend the affected sites despite exacerbation [19]. This indicates a tolerance system that depends neither on central tolerance nor Foxp3+ Treg cell-mediated peripheral tolerance. Tr1, Th3, and CD4+CD25-LAG3+ Treg cells may contribute to such a tolerance system.
5.5.7.9 Tr1, Th3, and LAG3 Treg Cells
Tr1 or Th3 cells are CD4+ T cells that produce IL-10 or TGF-β, respectively [152]. These subsets do not necessarily express Foxp3.
Tr1 cells are induced by antigen stimulation in the presence of IL-10 and vitamin D3 in vitro. Type I interferon, IL-27, and ICOS are reported to induce IL-10 producing T cells in a transcription factor c-Maf –dependent manner [104] (Fig. 5.7). IL-27 induces AhR, which activates c-Maf [5, 105]. Nasal administration of anti-CD3 antibody induces such AhR -dependent Tr1 cells in mice [158]. The ligand for AhR includes halogenated aromatic hydrocarbons such as dioxin that are famous as environmental pollutants with pleiotropic effects such as teratogenesis and immune suppression.
Th3 are induced in animal models for oral tolerance. Th3 may be a subset of pTreg cells.
LAG3 Treg cells are CD4+CD25-LAG3+ T cells that express IL-10 , but neither IL-2, nor IL-4, nor Foxp3 [97]. The suppressive function of LAG3 Treg cells is mediated by IL-10. LAG3 (lymphocyte activation gene-3) structurally resembles CD4, and binds to MHC-II with higher affinity than that of CD4, thereby attenuating activation of CD4+ T cells [20]. The cytoplasmic domain of LAG3 inhibits homeostatic expansion of T cells [157]. In humans and mice, expression of both LAG3 and CD49b is a marker for Tr1 cells [49]. Therefore, Tr1 cells—a subset originally induced in a test tube—may naturally exist in mice and humans as LAG3 Treg cells.
5.6 CD8 T Cells
(Summary) CD8 T cells are the principal effector cells that recognize antigens on MHC-I. CD8 T cells can produce inflammatory cytokines such as IL-17 and IL-22, and play a central role in contact dermatitis, drug eruption, graft versus host disease, and tumor immunity. Epidermal CD8 T cells constitute resident memory T (Trm) cells .
5.6.1 Characterization of CD8 T Cells in the Skin
CD8 T cells are called killer or cytotoxic T lymphocytes (CTLs) . They recognize antigens expressed on MHC-I on the infected cells. CD8 is a cell surface molecule that binds a conserved region of the MHC-I molecule. Engagement of the TCR activates the cytolytic machinery of CD8 T cells. The cytolytic factors include perforin, granulysin, and Fas ligand, which induce apoptosis of target cells. Skin-homing CD8 T cells express P-selectin ligands, E-selectin ligands, PSGL-1 (P-selectin glycoprotein ligand-1, CD162, a ligand for P-, E-, and L-selectin), CLA, CD43 (a ligand for E-selectin), and CD44 (a memory marker, a receptor for hyaluronic acid) [7, 11, 47, 82]. CD8 T cells occupy a central role in skin immune responses such as contact dermatitis, drug eruptions, graft versus host disease-mediated skin inflammations, and tumor immunity against skin tumors such as melanoma and squamous cell carcinoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








