Adverse effects
Diagnostic studies
Preventative intervention
Glucocorticoid-induced osteoporosis
Dual Energy Absorptiometry
(DXA scan)a
Calcium, vitamin D
Risk factor modificationb
Bisphosphonates
Denosumab
Teriparitide
Raloxifene
Hormonal therapy
Calcitonin
Osteonecrosis
X-rays
MRI
Early diagnosis and reduced weight bearing
Endocrine (Diabetes mellitus)
Regular glucose monitoring
Hemoglobin A1c
Diet and weight control
Oral anti-diabetic medications and insulin
Endocrine (HPA suppression)
Cosyntropin stimulation test
Slow tapering of glucocorticoids
Psychiatric
Discussion with patient/family
Antidepressants
Anxiolytics
Sleep aids
Cardiovascular (dyslipidemia, atherosclerosis, hypertension)
Lipid profile
Monitor blood pressure
Lipid-lowering agents
Anti-hypertensives
Infectious
PPD, Chest radiograph
Vaccinations
Ophthalmologic
Annual eye examination
None known
Gastrointestinal
Monitor CBC
Stool occult blood testing
Endoscopy
Chewable calcium carbonate with vitamin D supplement
Proton-pump inhibitor
H2-blocker
Table 13.2
Medications for the prevention and treatment of glucocorticoid-induced osteoporosis
Medication | Dosage | Route of administration |
|---|---|---|
Alendronate (Fosamax) | 70 mg/week | Oral |
Risedronate (Actonel) | 35 mg/week or 150 mg/month | Oral |
Ibandronate (Boniva) | 150 mg/month 3 mg/3 months | Oral Intravenous |
Zolendronic acid (Reclast) | 5 mg/12 months | Intravenous |
Denosumab (Prolia) | 60 mg/6 months | Subcutaneous injection |
Teriparitide (Forteo) | 20 mcg/day | Subcutaneous injection |
Osteonecrosis
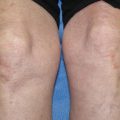
Osteonecrosis is a rarer but more insidious and debilitating complication of treatment with corticosteroids. Osteonecrosis occurs most commonly in patients taking prednisone doses of greater than 15 mg/day. High dose pulsed intravenous steroids, steroid dose packs, and intra-articular steroid injections have also been associated with the development of osteonecrosis [10]. Other risk factors for osteonecrosis include vitamin D deficiency, high alcohol consumption, history of venous or arterial thrombosis, chronic treatment with anticoagulant and anti-convulsant medications, and treatment with Depo-Provera. Osteonecrosis occurs most commonly in the hip, knee, and ankle joints. Osteonecrosis should be suspected in any patient being treated with glucocorticoids who complains of chronic joint pain. Plain radiographs are frequently normal in patients with osteonecrosis, thus MRI scanning may be necessary to make the diagnosis.
Metabolic and Endocrine Side Effects
Hyperglycemia and diabetes mellitus are potential complications of even low dose corticosteroids. Persons at the highest risk for these adverse effects include those with existing glucose intolerance, obese persons, the elderly, and patients with a significant family history of diabetes. Corticosteroid –induced diabetes usually responds to steroid dose reduction and may fully reverse after cessation of glucocorticoid use. In general, all patients in whom treatment with corticosteroids is expected to last greater than 30 days, should have their glucose levels checked prior to initiation of corticosteroid therapy and periodically thereafter. Fasting glucose levels greater than 125 mg/dl or random glucose levels greater than 200 mg/dl warrant hemoglobin A1c testing [11].
Long-term corticosteroid treatment also commonly causes hypothalamic-pituitary-adrenal (HPA) suppression with secondary adrenal insufficiency. Although not fully understood, this has been observed to occur after the use of as little as 10 mg/day of prednisone for 4–6 weeks [11]. HPA suppression may occur more rapidly with higher doses of corticosteroids or with twice-a-day dosing. Symptoms of adrenal insufficiency after corticosteroid use include arthralgias, myalgias, fatigue, nausea, vomiting, and hypotension. In cases of suspected glucocorticoid-induced adrenal insufficiency, a cosyntropin stimulation test may help confirm the diagnosis [11].
Psychiatric Side Effects
Treatment with corticosteroids frequently induces or exacerbates many psychiatric conditions including depression, anxiety, and disrupted sleep. Psychosis is also an uncommon but well described potential complication of corticosteroids [12]. Memory impairment, particularly in older patients, can occur at doses as low as 5 mg/day. Psychiatric side effects are significantly more likely to occur with twice daily dosing and with dosages above 30 mg/day [13]. Given the fact that depression, anxiety, and disrupted sleep are already very common in patients with chronic autoimmune diseases, clinicians may need to add or intensify treatment with antidepressants, anxiolytics, and sleep aids during treatment with corticosteroids.
Cardiovascular Side Effects
Corticosteroid use is associated with an increased risk of serious cardiovascular events and hypertension. Treatment with prednisone doses ≥7.5 mg/day was associated with a greater than 2.5-fold increased risk of cardiovascular events in a population-based study of more than 150,000 persons [14].
Chronic corticosteroid use has been associated with dyslipidemia and atherosclerosis in several conditions including systemic lupus erythematosus (SLE), asthma, and organ transplant recipients [15–17]. In lupus patients, the adverse lipid profile effects appear to occur at prednisone doses greater than 10 mg/day [15].
Increased hypertension has been observed in about 30 % of patients using glucocorticoids [18]. Fluid retention has been postulated to partially account for this observation. As with dyslipidemia, the risk of hypertension with glucocorticoid use appears to be greatest with doses greater than 10 mg/day. Lipids and blood pressure should be monitored and aggressively treated in patients being treated with chronic glucocorticoids with either a personal or family history of hypertension, cardiac disease, or renal disease.
Infectious Disease Side Effects
Corticosteroids increase susceptibility to many types of infectious agents. This issue has been well researched in patients with rheumatic diseases. Using the National Data Bank for Rheumatic Diseases in the USA, over 16,000 patients were followed for 3.5 years and observed for hospitalized pneumonia. Patients receiving glucocorticoid therapy had hospitalized pneumonia rates 1.7 times greater than those not receiving glucocorticoids. The study even identifies risk at doses less than 5 mg/day (hazard ratio 1.4) and even higher risk at 10–15 mg/day (hazard ratio 2.3). The increased rate of serious infections was more pronounced during the first 90 days after initiation of treatment with glucocorticoids [relative risk (RR) 2.99] [19]. A cohort study of 15,597 RA patients from a Medicare beneficiary database found that glucocorticoid use doubled the rate of serious bacterial infections compared with methotrexate use (RR 2.1), with a dose–response relationship for doses greater than 5 mg/day: (5 mg/day, RR 1.34; 6–9 mg/day, RR 1.53; 10–19 mg/day, RR 2.97; 20 mg/day, RR 5.48) [20].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree