Fig. 8.1
Straight end-to-end anastomosis
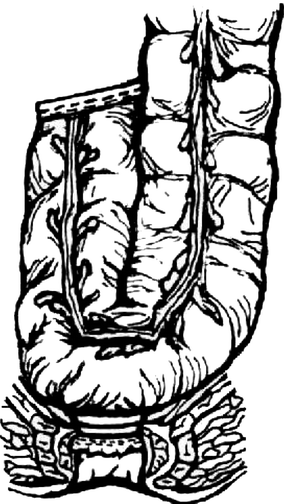
Fig. 8.2
Colonic J pouch anastomosis
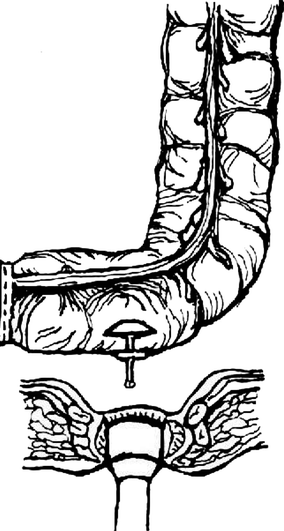
Fig. 8.3
Side-to-end anastomosis
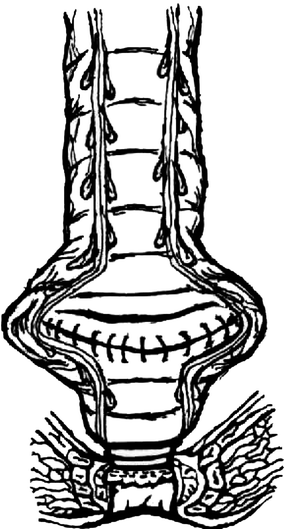
Fig. 8.4
Transverse coloplasty with end-to-end anastomosis
Anastomotic Complications
A Cochrane meta-analysis [9], which summarizes 866 patients from 8 randomized trials, found no statistically significant differences in leak rate when the colonic J pouch was compared with a straight anastomosis (odds ratio [OR], 0.71; 95 % confidence interval: 0.20–1.66). Similarly, a systematic review of randomized and nonrandomized studies, the majority of which were prospective, by Heriot et al. [10] summarized data from 154 patients in 3 studies and found an OR of 0.33, which was not statistically significant for anastomotic leaks, favoring the colonic J pouch. No difference was seen in anastomotic strictures in Heriot et al.’s summary of results of 66 patients from 2 studies. Some authors have theorized that the proximal limb of the anastomosis is better vascularized when a side colotomy is used for the colonic J pouch or for a side-to-end anastomosis because of a less disrupted mesentery. In a meta-analysis of seven randomized trials by Koh et al. [11], the risk ratio for anastomotic leak was 0.36 (95 % confidence interval: 0.12–1.08), which indicated a marginally higher risk of leak for a straight anastomosis; however, this view was primarily influenced by a single randomized trial of nearly 100 patients by Hallböök et al. [12] in which 15 % of patients with a straight anastomosis had symptomatic leaks compared with 2 % of patients with a colonic J pouch (P = 0.03). Other studies show an 8–16 % leak rate for the straight coloanal anastomosis [13–15] and an 8–9 % [13–15] rate for the colonic J pouch.
When comparing the colonic J pouch with the side-to-end anastomosis, the Cochrane meta-analysis [9] found no differences in anastomotic leaks in 215 patients from 3 randomized trials. Leak rates for the side-to-end construction range from 7 to 10 % [16, 17]. In a study by Jiang et al. [18], one patient (3.5 %) developed necrosis of the side limb, whereas in the well-quoted Machado et al. [17] study of 2003, 4 % of patients randomized to the side-to-end construction developed an anastomotic stricture. A randomized trial of 40 patients designed to evaluate a 3-cm-long versus 6-cm-long limb of the side-to-end anastomosis found a 10 % colonic stump leak in the longer limb group compared with no stump leaks in the short limb group [19].
Transverse coloplasty has been perceived to have a higher anastomotic leak rate on the basis of one randomized trial by Ho et al. [20], which had a mean anus-to-anastomosis distance of 3.4 cm and routinely used a defunctioning loop ileostomy. Leaks in this study occurred in 16 % of 44 coloplasty patients compared with no colonic J pouch leaks (P = 0.01), with all of the leaks noted in the anterior aspect of the coloanal anastomosis distal to the coloplasty. Ho et al. hypothesized that increasing the distance of the coloplasty incision from the distal margin may preserve the blood supply to the end-to-end anastomosis and decrease the risk of leak; in their view, the longitudinal incision should be made at least 4 cm above the distal transected end. In agreement with this concept, the Cleveland Clinic proposed a 4- to 6-cm distance from the colostomy to the distal margin for construction of a coloplasty [21]. In this respect, retrospective data from the Cleveland Clinic has shown an anastomotic leak rate of 4.8 % and a stricture rate of 4.8 % in the stapled coloplasty anastomosis, with a high (28.5 %) stricture rate in patients undergoing a hand-sutured coloplasty anastomosis [22]. In this study by Remzi et al., it is likely that a low hand-sutured coloanal anastomosis, rather than the actual technique of rectal reconstruction itself, contributed to significant anastomotic complications. In this regard, hand-sutured colonic J pouch anastomoses (of which there were 9 in this report) resulted in a 44 % leak rate and a 22 % stricture rate. In the more recent multicenter, randomized trial from another group at the Cleveland Clinic [23], coloplasty patients had an 8.5 % anastomotic separation rate and a 12.8 % stricture rate if they were ineligible for a J pouch compared with a 4.6 % anastomotic separation rate and a 3.8 % stricture rate if they were eligible for a J pouch.
A Cochrane meta-analysis showed no statistical difference in leak rates when comparing the J pouch to coloplasty in 158 patients from 3 randomized trials [10], a finding endorsed by a more recent meta-analysis performed by Liao and colleagues [24] that analyzed a total of 316 patients collated from 4 randomized trials. This group reported an overall leak rate of 5 % in the colonic J pouch group and 10 % in the transverse coloplasty group; however, the OR of 0.50 did not reach statistical significance. Anastomotic stricture rates between these two techniques were not different in this later meta-analysis, occurring in 5.5 % of colonic J pouch patients and 4 % of transverse coloplasty patients.
In summary, multiple studies seem to show that the rates of anastomotic leakage tend to be lower for the colonic J pouch technique compared with other techniques, although many do not reach statistical significance. In contrast, because data concerning anastomotic stricture rates is limited, no specific reconstruction technique is favored. The coloplasty technique may be used selectively, despite these inferior results, as a substitution for construction of a colonic J pouch when the J pouch is technically difficult to perform, usually because of a short mesentery or a narrow pelvis. Physiological volumetric parameters volumetric parameters assessing neorectal capacity and sensation do not seem to differ between the two approaches [25]. Despite the increasing popularity of the use of pouch reconstruction for low rectal cancer compared with a straight colorectal or coloanal anastomosis and despite concerns in some series regarding the anastomotic leakage rates, there is still controversy regarding the most suitable part of the colon for pouch use, the pouch size recommended, the optimal level of anastomosis, the obviation in some patients of postoperative evacuatory difficulty, its long-term (>2 year) outcome, and its surgical indication in elderly patients [26].
The Assessment of Bowel Function After Rectal Reconstruction
Defecatory function is relatively difficult to quantify and measure for benign and malignant conditions. Multiple aspects of function require evaluation, but a single measure, score, or survey has not yet evolved to adequately summarize anorectal function. Two instruments have been methodically designed and rigorously validated but have not yet been widely adopted in research. The Colorectal Functional Outcome Questionnaire has been developed and validated by Bakx et al. [27], and the Memorial Sloan Kettering Cancer Center Bowel Function Instrument, which was designed by Temple et al. [28] specifically for use in patients with rectal cancer. Commonly reported components of bowel function in the existing literature are listed in Table 8.1. Bowel frequency, or the number of bowel movements per day, is the most consistently reported parameter.
Table 8.1
Symptoms related to anorectal function
Bowel frequency | |
Fecal incontinence | |
Incomplete evacuation | |
Clustering/fragmentation | |
Fecal urgency | |
Impaired anal sensation | Ability to distinguish between flatus and stool |
Dietary modification to control patterns of defecation | |
Use of pads | |
Use of antidiarrhea medication | |
Use of laxatives or enemas |
Fecal incontinence after surgery has been variably reported and may refer to fecal staining, incontinence of flatus, loose stool, or formed stool. Studies may use scoring systems to summarize the severity and frequency of incontinence. Of these, the Kirwan-Fazio score [29] is the simplest score; it has four levels, namely, no incontinence (I), incontinence to flatus (II), incontinence to flatus and liquid stool (III), or frequent major soiling (IV). The Hallböök score [12] and Fecal Incontinence Severity Index (FISI) account for the frequency of varying degrees of incontinence, with a higher score indicating more severe symptoms [30]. The FISI scoring system is shown in Table 8.2.
Table 8.2
Fecal Incontinence Severity Index (FISI)
Two or more times per day | Once a day | Two or more times per week | Once a week | One to three times per month | |
|---|---|---|---|---|---|
Gas | 12 | 11 | 8 | 6 | 4 |
Mucus | 12 | 10 | 7 | 5 | 3 |
Liquid stool | 19 | 17 | 13 | 10 | 8 |
Solid stool | 18 | 16 | 13 | 10 | 8 |
Incomplete evacuation refers to the reporting of a consistent inability to empty the bowel during a specific period of time (for example, >15 min). Clustering (also described as fragmentation) is the perceived completion of defecation with a subsequent need to evacuate again, either once or several times, soon thereafter. Fecal urgency is the sensation of an immediate need to evacuate with the threat of fecal incontinence. Urgency can be assessed by asking the patient how long they are able to defer defecation. Impaired anal sensation is the inability of a patient to distinguish stool from flatus and can be assessed by asking the patient whether he or she is able to release flatus “safely.” Finally, other measures of defecatory function inquire about activities that compensate for symptoms, such as dietary modification or restriction and the use of pads, antidiarrheal medications, laxatives, or enemas. Some studies report responses to queries about general interferences with lifestyle, such as whether patients are “hindered from leaving home,” “dissatisfied with bowel function,” or “content with postoperative continence.”
Radiation
Preoperative and postoperative external beam radiation has been shown to improve cancer-specific outcomes, particularly local recurrence rates, after surgical resection of rectal cancer. Overall and disease-free survival, however, has not been consistently shown to improve with radiation. In addition, radiation is suspected to contribute to major short- and long-term complications such as poor healing and postoperative small bowel obstruction. In the case of sphincter-preserving surgery, preoperative and postoperative radiotherapy have long-lasting adverse effects on bowel function. The mechanism of these effects is thought to be related to fibrosis in the pelvic soft tissues, perirectal nerves, and anal sphincter.
Preoperative radiation, either short-course (25 Gy, usually over 5 days) or long-course chemoradiation (50 Gy, usually over 4–6 weeks), is associated with problematic fecal function in long-term follow-up. In an analysis of 64 patients from the Stockholm 1 and 2 trials [31], 21 of whom had been treated with preoperative radiotherapy (25 Gy), at a mean of 14 years’ follow-up, irradiated patients had increased bowel frequency and more problems with fecal incontinence (57 % vs. 26 % of nonirradiated patients; P = 0.01). The mean anastomotic height in this study was 10 cm from the anal verge. As part of the Dutch total mesorectal excision study, 362 patients were surveyed 5 years after low anterior resection [32]. Preoperatively irradiated patients (25 Gy) had worse bowel frequency (3.69 vs. 3.02 per day; P = 0.011), fecal incontinence (62 % vs. 38 %; P < 0.001), and a more common use of pads; these were more prominent in patients with low tumors (5–10 cm from the anal verge). A larger proportion of irradiated patients reported an impact of overall bowel function on their daily activities (34 % in patients receiving radiation therapy vs. 22 % in patients who did not receive radiation therapy; P = 0.01). Detailed long-term data on anorectal function after preoperative long-course chemoradiation is somewhat lacking. Patients studied in the Polish rectal cancer trial were randomized to either preoperative long-course chemoradiation or short-course radiation. At 12 months’ follow-up, bowel function was equally poor in both groups, with incontinence in 42 % of “short-course” patients and 50 % of “long-course” patients [33]. Fecal urgency was reported in approximately 60 % of patients and clustering was reported in approximately 90 % of patients in this study.
Long-term follow-up concerning surviving patients after postoperative radiation shows that they commonly have chronic diarrhea, fecal urgency, clustering, and incontinence compared with patients who did not receive radiation. Kollmorgen et al. [34] reported these findings in 41 patients who received postoperative chemoradiation at a median of 40 months’ follow-up after rectal resection, and Lundby et al. [35] found similar symptoms in 15 patients who were followed in 17-year follow-up from a Danish randomized trial that commenced in the 1980s and was reported in 2005.
A secondary analysis of data from the randomized trial of coloplasty versus J pouch or straight anastomosis in low (≤4 cm from the dentate line) rectal cancers conducted by Fazio et al. [23] was performed by Parc et al. [36] to evaluate the effect of neoadjuvant therapy on bowel function. Of the 364 patients enrolled in the trial, 58 % underwent preoperative radiotherapy, and 88 % of those also received chemotherapy. The use of neoadjuvant therapy was not randomly assigned. Patients who had preoperative radiotherapy had more bowel movements per 24 h at 4 months after surgery, with more frequent night-time bowel movements at 4 and 12 months. By 4 and 24 months postoperatively, urgency was more frequent in patients who had had preoperative radiotherapy; however, the authors unexpectedly noted that fecal continence was better in the patients who had preoperative neoadjuvant therapy at 24 months’ follow-up.
Functional Results After Reconstruction
Straight Anastomosis Compared with Colonic J Pouch
The colonic J pouch has been studied extensively compared with the straight end-to-end anastomosis (this is also covered in Chap. 9). Overall, most studies favor the use of a colonic J pouch [26]. Few studies provide long-term results (2-year follow-up or longer), and they show fewer differences in defecatory function between the two techniques.
Bowel Frequency
Bowel frequency is improved in short-term (<12 months) follow-up in patients with a colonic J pouch. The Cochrane meta-analysis by Brown et al. [9] found that five of seven randomized trials showed statistically fewer bowel movements per day in the groups who had a colonic J pouch. This difference persisted at “medium-term” follow-up (8–18 months). The mean distance of the anastomosis from the anal verge was less than 5 cm in all trials except one. The systematic review by Heriot et al. [10] evaluated randomized and nonrandomized studies, the majority of which were prospective. Fewer bowel movements per day were seen with the J pouch, with a weighted mean difference of 1.88 per day at 6 months (P = 0.011) and 1.35 per day (P = 0.001) at 12 months of follow-up. A Cochrane meta-analysis by Ho et al. [37] identified only two trials with 24 months follow-up, one of which showed a difference favoring the J pouch. In this trial, 3 of 16 patients with a J pouch (19 %) compared with 7 of 19 patients with a straight anastomosis (37 %) had more than four bowel movements per day. The Heriot systematic review based on five studies encompassing 331 patients found a difference of 0.74 bowel movements per day (P = 0.01) at 24 months of follow-up.
Fecal Incontinence
Only one study [12] in the Cochrane meta-analysis [9] reported administration of preoperative radiotherapy, and in most of the trials analyzed only small proportions of patients received postoperative radiation. The population of patients in this meta-analysis, therefore, is unlikely to suffer adverse effects on continence as a result of the type of radiation therapy that is commonly utilized today. Fecal incontinence was similar in both groups, with the exception of two of seven trials that showed less fecal incontinence in J pouch patients over medium-term (8–18 months) follow-up. Hallböök et al. [12] found that the fecal incontinence score was significantly worse in patients with straight anastomosis versus a J pouch at both 2 months and 1 year. In a retrospective series of rectal resection patients – 85 % of whom had cancer – Joo et al. [38] reported statistically worse Jorge-Wexner incontinence scores in the straight coloanal anastomosis group at 6 months (mean, 5.3 vs. 2.0; P < 0.05), but these differences became nonsignificant at 1 and at 2 years of follow-up. Incontinence was not severe in either group in the long-term follow-up, with most patients rating incontinence as “rare” or “occasional.”
Fecal Urgency
Many studies find that patients are less bothered by fecal urgency if they have a colonic J pouch reconstruction. In the Cochrane meta-analysis by Brown et al. [9], less fecal urgency was identified in J pouch patients at short-term follow-up in three of six trials. In medium-term follow-up, only one of seven trials found a difference. There were no differences discovered in long-term follow-up. The systematic review by Heriot et al. [10] found that fecal urgency was less common in J pouch patients at 6 months as assessed in 8 studies (21 % vs. 51.4 %; P = 0.001), at 12 months as assessed in 15 studies (8.7 % vs. 30.3 %; P < 0.001), and not different in three studies with 2-year follow-up.
Other Measures of Defecatory Function
Other Considerations
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








