Type of bias
Description
Relevant domains in the collaboration’s “risk” of of bias tool
Selection bias
Systematic differences between baseline characteristics of the groups that are compared
• Sequence generation
• Allocation concealment
Performance bias
Systematic differences between groups in the care that is provided, or in exposure to factors other than the interventions of interest
• Blinding of participants and personnel
• Other potential threats to validity
Detection bias
Systematic differences between groups in how outcomes are determined
• Blinding of outcome assessment
• Other potential threats to validity
Attrition bias
Systematic differences between groups in withdrawals from a study
• Incomplete outcome data
Reporting bias
Systematic differences between reported and unreported findings
• Selective outcome reporting
10.3 Importance of Randomized Controlled Trials (RCTs)
RCTs are recognized as the “gold standard” to assess the effectiveness of new interventions but there is some disagreement [8, 9]. Nevertheless, a series of RCTs in dermatology have been collated into an online database of dermatological eczema trial results [10]. It is now considered unsafe to rely on data from a single RCT, not because it is unsafe for the patient but rather because it may not be well controlled. Nevertheless, if a single RCT is large enough and well controlled, it should provide adequate evidence to support the tested hypothesis. However, there can be no doubt that systematic reviews (SRs) that collate information from many studies provide a more reliable body of evidence. SRs that connect data from different trials are referred to as meta-analyses, and often reveal answers to questions that may be overlooked in separate studies [3, 11]. They are an essential tool for providing collated trial results to healthcare professionals as they identify the best therapy for a specific disease. SRs minimize bias by considering all of the available literature (Fig. 10.1). A significant drawback of SRs is that they require substantial work to conduct and crucially keep them up to date, despite their admirable and exhaustive assessment of relevant data.
When designing a clinical trial it is important to decide whether it can be linked to other studies and thus obtain a general consensus about the effectiveness of a new intervention.
10.3.1 Issues to Consider When Designing and Implementing a Clinical Trial in Dermatology
It goes without saying that clinical trial design should follow standard good clinical practice for the benefit of all subjects involved. In the final analysis, the duty of patient care must hold sway over all other considerations even if it is to the detriment of the study. It is vital that sound methodological principles are implemented from the outset and it is strongly advised that the input of a statistician be sought. Fundamental issues that must be considered include the principle of clinical equipoise, selection of participants, type of trial, and power calculations. It is essential to have hard endpoints in the trial (e.g., objectively measurable, clinically relevant endpoints) and not to do preliminary sub-analyses if possible. The trial must be designed in such a way that unequivocal conclusions can be drawn. An independent advisory board should hold sway over the conduct of the clinical trial to curb any “over enthusiasm” of the personnel conducting the trial. A current example of clinical equipoise in dermatology is the treatment of bullous pemphigoid with either prednisolone or tetracyclines [12].
10.3.2 Methodological Considerations in Clinical Trials
A common type of clinical trial is conducted to test the effectiveness of an established treatment regime over a newer therapy. A flow chart of such a trial is shown in Fig. 10.2 [13].
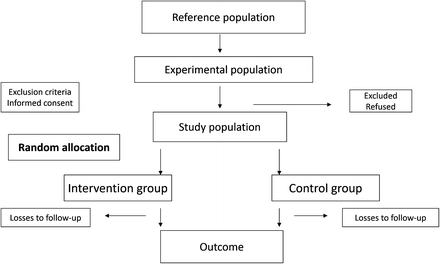

Fig. 10.2
Flow chart of a clinical trial
This chart provides a graphical picture of a trial plan from beginning to end. This is a good example of a clinical trial for several reasons:
There is a clearly defined primary and secondary objective
Well-documented criteria for selection of participants
The interventions are clearly described
Implementation of randomization and blinding (masking)
Appropriate sample size and statistical analysis
Assessment of the cost-effectiveness of the interventions
Next, some of the factors that should be considered before embarking on a clinical trial are discussed.
10.4 Ethical Considerations
Ethics in human research were considered only after the dreadful medical experiments carried out by the Nazis came to light. As a result, ten basic principles of human research were formulated in the Nuremberg Code of 1949 [14] that was later developed into The Declaration of Helsinki and accepted by the World Medical Association [15]. A phrase introduced in 1987 by Freedman [16], “clinical equipoise” was coined to describe a medical dilemma that can arise during a clinical trial. A trial starts with the assumption that it is not known if the intervention being tested will be more effective than the existing one. The problem occurs when it is obvious that one treatment is much more effective than another. Thus, there is an ethical necessity for the investigating clinicians to provide the improved treatment to all participants.
10.5 Importance of Informed Consent of Participants in a Clinical Trial
Informed consent is mandatory and the consent form must clearly state [17, 18]:
It is a research study including the purpose, duration, risks, benefits, and alternatives to the intervention being studied
That participation is voluntary
Confidentiality will be rigorously observed
Contact details and information will be available if a subject has questions or concerns about the study
Interestingly, these safeguards are not flawless. This is because the clinician running the trial necessarily has incomplete knowledge regarding the risks and benefits of the intervention because they are not known.
10.6 Integrity of Findings
It is important to realize that there is some evidence that industry-funded trials have tended to report favorably on new drugs (human nature being what it is, loyalty to the sponsor, etc.). Therefore, all trial researchers involved in a trial must be completely impartial.
10.7 Statistical Considerations
It is vital to collaborate with an experienced statistician who will help with the design of the trial and assist in selecting an achievable outcome. A statistician’s input is invaluable in deciding many factors including an appropriate sample size, randomization protocols, and the overall analysis of trial results following data collection. Bhardwaj and colleagues [19] laid down sound statistical criteria that should be considered in the design of a dermatological clinical trial. They concluded that studies that claim clinical relevance may actually lack sufficient statistical significance to make the conclusion true and, conversely, that a study purporting to show a statistically significant difference between two interventions may lack expediency. The power of a statistical study is the probability of detecting a difference when one exists. Bhardwaj and colleagues [19] explained the importance of power by using specific examples taken from the dermatological literature. It is vital to understand the direct relationship between sample size and power when drawing conclusions. The failure to detect a clinically important difference between two groups can occur as the result of inadequate sample size; that is, inadequate power [19–21]. This difficulty will most likely arise in studies of rare medical conditions, but it can also be a hindrance to studies involving more common ailments. As the power of a statistical study increases, the ability to detect progressively smaller differences also increases. Therefore, the perception of a particular study having too much power must be considered. In contrast, studies with very large sample sizes may detect statistically significant differences that are not clinically relevant [19].
Inset 10.1
Statistical Methods
In the 1930s, Sir Ronald Aylmer Fisher introduced statistical methods to research including randomization, and analysis of variance. Around the same time, Torald Sollman added placebo arms and blinding to studies. One of the challenges of increasingly complex clinical trials and pooled trials is reproducibility. One of the best ways to maximize validity and reproducibility is to minimize statistical bias and statistical error. Scientific journals are now adding statisticians to their editorial boards. Their task is to rigorously scrutinize the statistical methodology of high profile studies.
The concept of power in a clinical trial refers to the probability of detecting a difference between study groups when a true difference exists. This statistical power is undermined if the number of participants in each study is too small to identify important differences that may exist. In contrast, a study can be overly large and spuriously identify differences that are not actually clinically significant [19]. Thus the purpose of statistical analysis is to determine whether the findings are due to chance rather than to genuine differences between the treatments. What could be worse than carrying out a large-scale clinical trial, only to be told that there were fundamental flaws in its design and therefore the conclusions.
To recap, a small study that claims clinical relevance may lack sufficient statistical power to justify its conclusions. Conversely, authors of a study may speak of the statistical significance of a treatment effect that has little, if any, clinical efficacy. Therefore, when evaluating the validity of a study, the intelligent reader must consider both the clinical and statistical significance of the findings. There is a wealth of literature available to the interested reader that describes the mathematical basis of the statistics required for a clinical trial [19–24]. Important factors to consider are the values of the type one error rate, α, and power, 1 − β (i.e., how mathematically exacting the study will be), as well as the expected improvement to be detected, which will determine the sample size of the study. Reliable efficacy data are required because if the estimate of the efficacy of the comparator treatment is wrong, the results of the study may be underpowered, hence failing to produce the intended results. In the study highlighted in Fig. 10.2, a total of 140 patients (randomized 1:1 to prednisolone or ciclosporin) gave the study about 80 % power [13]. This sample size also allowed for an approximate 10 % loss of patients to follow-up after 6 weeks of the trial. The outcomes of any trial, whether objective or subjective, must always be reliable and provide meaningful measures. Statistical techniques that can be used to analyze trial outcomes include logistic regression for dichotomous endpoints, Poisson regression for rates, Cox regression for time-to-events, and linear regression for continuous measures e.g., the weights of participants [1].
10.8 Selection of Participants
For a clinical trial to be successful the selection of an appropriate study population is crucial. Even if all participants volunteer for the intervention, the enrolled cohort may differ from the general population. This can inadvertently lead to a bias in selection known as “volunteer bias.” Many factors may be involved including the trial criteria for inclusion, intrinsic attributes of the subjects or deliberate exclusion of a potential participant because the investigator subjectively believed that their overall prognosis might be detrimental to the trial. Without a suitable cohort of participants, the measure of the success of an intervention may not translate into useful new clinical therapy [2].
10.9 Write a Detailed Clinical Trial Observational Plan
One important methodological consideration is to have a well-thought-out protocol available. Its purpose is to provide all personnel involved in the trial with documentation that should:
guide the conduct of the trial
give participants a detailed description of the methods used
inform review boards of predefined safeguards to protect the safety of the participants
permit potential funding bodies to assess the research proposal
provide peer reviewers with a report of pre-specified methods designed to evaluate potential bias [25]
To fulfill the above purposes, the protocols should be detailed and transparent. Often protocols do not adequately describe methodological details such as allocation concealment, primary outcomes, power calculations, and the roles of sponsors and investigators in the trial. Apposite randomization rests on adequate allocation concealment, which ensures that clinicians and trial participants are unaware of the treatments. Without allocation concealment, random allocation sequences can be circumvented undermining the crucial unbiased nature of RCTs [26]. Selection bias refers to the possible differences between baseline characteristics in the groups under comparison. Investigators should devote appropriate resources for allocating interventions to participants on the basis of some chance (random) process and report their methods clearly, avoiding nonrandom methods of allocation. Adequate generation of the randomization sequence takes little effort but undoubtedly increases the degree of scientific accuracy and the credibility of the trial. Inadequate allocation concealment leads to either an underestimation or an overestimation of the efficacy of the treatment under investigation. If these safeguards are not implemented, exaggerated descriptions of the effectiveness of interventions can be made. A lack of transparency and incomplete description of methods makes critical assessment of trials difficult. Therefore, the Delphi consensus was developed to provide useful information to guide the development of trial protocols [25].
10.10 Assessment of Data Quality and Guarding Against Bias
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree