Fig. 12.1
Breast duct with normal duct cells (1), with ductal hyperplasia (2), with atypical ductal hyperplasia (3), with carcinoma in situ (4), and with microinvasive ductal carcinoma (5)
Because of the difficulties in separating low-grade DCIS from atypical intraductal hyperplasia, a different classification has been proposed where these proliferations are classified as ductal intraepithelial neoplasia (DIN), or also as mammary intraepithelial neoplasia, ductal type. The latest WHO Classification of Tumours of the Breast [1] includes this terminology (Table 12.1), however it still needs to be fully clarified. This could be the result of the failure to introduce new diagnostic criteria useful to reduce the inter-observer variability and the diagnostic difficulties between ADH and some cases of low-grade DCIS. Even now, for purposes of clinical management, when the DIN terminology is used, the traditional terminology should be mentioned as well.
Table 12.1
Classification of DIN in comparison with traditional classification
Traditional terminology | Ductal intraepithelial neoplasia (DIN) terminology |
|---|---|
Flat epithelial atypia | DIN 1a – ductal intraepithelial neoplasia grade 1a |
Atypical ductal hyperplasia (ADH) | DIN 1b – ductal intraepithelial neoplasia grade 1b |
Low-grade DCIS (DCIS G1) cribriform or micropapillary | DIN 1c – ductal intraepithelial neoplasia grade 1c |
Intermediate-grade DCIS (DCIS G2) cribriform or micropapillary with necrosis or atypia or other types | DIN 2 – Ductal intraepithelial neoplasia grade 2 |
High-grade DCIS (DCIS G3) with or without necrosis (comedo-type DCIS) | DIN 3 – ductal intraepithelial neoplasia, grade 3 |
This approach obviates the current separation of ADH and low-grade DCIS into two very drastically different categories of noncancer and cancer without interfering with appropriate management of the various lesions. A more extensive use of this separation of terms would have many advantages in communication with the patient. The forms of noncancer would elicit less anxiety by the patient, while the forms of cancer would be considered the way they are perceived, i.e. as invasive.
The above classification (Table 12.1) comprises the traditional DCIS subtypes based primarily on architectural pattern: micropapillary, papillary, solid, cribriform, and comedo. Well-differentiated categories of DCIS are of the non-comedo subtype and poorly differentiated of the comedo subtype (Table 12.2).
Table 12.2
Subtypes of DCIS: histological characteristics, calcifications features, and prognosis
Subtypes | Grade | Necrosis | Calcification | Prognosis |
|---|---|---|---|---|
Comedo | High | Extensive | Branched | Worse |
Intermediate | Intermediate | Limited | Limited | Favourable |
Non-comedo: | Low | Absent | Inconsistent | Solid: favourable |
Solid | ||||
Micropapillary | Micropapillary: good Cribriform: very good | |||
Cribriform |
Non-comedo subtypes of DCIS are low nuclear grade and have a positive ER status (ER+). Mitoses are relatively infrequent and the prognosis is good. In the non-comedo category, cribriform carcinoma has a better prognosis; micropapillary carcinoma has a favourable prognosis, while solid carcinoma is less favourable due to its intermediate grade. The developmental pathways for low- and intermediate-grade DCIS appear different from that for high-grade DCIS.
Comedo-type DCIS has high nuclear grade, shows pleomorphism, and has abundant central luminal necrosis. 80 % of comedo lesions are aneuploid, are ER negative, have a high rate of HER2 overexpression, and express p53 mutations. Moreover, they are associated with greater tumour size, increased incidence of multicentricity and microinvasion, and a higher rate of local recurrence. In conclusion, comedo-type DCIS has a worse prognosis.
DCIS proliferation in the ductal system has a propensity toward longitudinal rather than radial growth. Due to the complexity of its presentations, many terms have been coined to outline the unpredictable localisation, architectural characteristics, invasive potential, and prognostic significance of DCIS.
Multifocal DCIS. Multifocality is another important characteristic of a majority of DCIS. Actually, upon closer evaluation by three-dimensional reconstructions of the cross-sectional segments, almost all foci seemingly disconnected are in essence unifocal, harbouring disease arising from convolutions of the same duct system.
Confinement of DCIS to multiple TDLUs (multifocality) without extension into the major duct system would imply an earlier stage in the development and progression of DCIS, but current sampling and processing methods in no way could distinguish multiple TDLU involvement from duct progression. Furthermore, DCIS lesions that have extended some distance toward the nipple are more likely to develop subsequent recurrences or invasive carcinoma compared with those confined to the TDLU. Obviously, it is very important to assess how far along this route (along the major duct and toward the nipple) the DCIS has already extended at the time of initial resection.
Multicentric DCIS, on the other hand, refers to foci of disease present in different sectors of the breast arising simultaneously in separate ductal systems. On average, 30 % of cases are believed to be multicentric.
Microinvasive DCIS has been defined by the American Joint Committee on Cancer (AJCC) as the extension of cancer cells beyond the basement membrane into adjacent tissues with no focus more than 1 mm in greatest dimension. Lesions fulfilling this criterion are staged as pT1mic, a subset of pT1 BC. It is helpful to consider that in the presence of multiple foci of microinvasion, only the focus with the largest dimension is used to classify the lesion and the sizes of individual foci are not added together. However, this aspect is still controversial.
The importance of the structure and activity of the basement membrane is increasingly recognised – a complex lattice-like structure lying between stroma and epithelium, with a complex paracrine pathway between stromal, myoepithelial, and epithelial cells.
Extensive intraductal component (EIC) is referred to invasive BC with an additional extra-tumoural focus of DCIS. EIC is properly defined as intraductal carcinoma occupying more than 25 % of the area encompassed by an invasive tumour and extending beyond the infiltrating edge of the tumour into the surrounding breast tissue.
Paget disease (see “Paget Disease”, Sect. 14.1) is a less common presentation of BC clinically characterised by eczematous, scaly skin at the nipple-areolar complex. It is associated with underlying (invasive and/or in situ) disease in 97 % of cases; therefore, it is important to consider Paget disease in any patient presenting with a persistent nipple-areolar complex abnormality.
Other considerations about multiple (multifocal and multicentric) BC are discussed in Sect. 15.1.
12.1.2 Diagnosis
Mammography. DCIS is often detected mammographically as microcalcifications. Currently, about 20–25 % of screening-detected malignancies are DCIS. Calcium deposition usually occurs in the areas of rapid growth and necrosis, leading to its typical mammographic appearance.
In mammograms with high-grade, comedo-type DCIS, calcium depositions resulting from increased necrosis are radiologically intense and often linear and branching. Low- and intermediate-grade DCIS are more likely to be discontinuous with gap areas typically small at <5 mm in 80 % of cases.
Core or surgical biopsies are the only reliable means in diagnosing DCIS, while fine-needle aspiration (FNA) should not be performed and moreover cannot distinguish DCIS from invasive carcinoma.
Frozen section has no role in the intra-operative diagnosis of non-palpable foci of microcalcifications or in the evaluation of margin status during glandular resection. This approach limits the ability of pathologists to evaluate the lesion overall.
Histology. DCIS may appear in different histological variants with specific cytonuclear, architectural, and molecular pathological features that can be recognised only by an expert histologist. The main differences between DCIS and infiltrating carcinoma are the disruption of the basal membrane and the way DCIS preserves the ductal/lobular anatomy.
DCIS accounts for about 80–85 % of non-invasive carcinomas. Lobular carcinoma in situ is not only less common (about 15–20 %) but also has different characteristics as compared to DCIS (Table 12.3).
Table 12.3
Features of ductal and lobular carcinoma in situ
DCIS | LCIS | |
|---|---|---|
Average age | Late 50s | Late 40s |
Menopausal status | 70 % postmenopausal | 70 % premenopausal |
Clinical signs | Breast mass or not, Paget disease, nipple discharge | None |
Mammographic signs | Microcalcifications | None |
Biomarkers | Comedo-type: ER negative, HER2 positive, high Ki67 | ER-positive, HER2 negative, low Ki67 |
Risk of subsequent carcinoma | 30–50 % at 10–20 years | 25–30 % at 15–20 years |
Site of subsequent invasive carcinoma | Same breast 99 % | Same breast 50–60 % |
Contralateral breast 1 % | Contralateral breast 40–50 % |
12.1.3 Treatment
The great pathologist David Page, in one of his most popular paper (1996), defined DCIS as understanding the misunderstood stepchild (that) has lagged behind our understanding of other elements of breast cancer. Although non-invasive, it is clearly a malignant disease and recurs in about 33 % of cases within 10–13 years if treated with excisional biopsy alone. The recurrence, if it occurs, is invasive carcinoma in 50 % of cases. When axillary node dissection has been performed, metastases have been found in <3 % of DCIS cases. When mastectomy has been performed, the disease is often found to be multicentric (additional CIS lesions >2 cm away from the main lesion).
SURGERY. Surgical treatment of DCIS is aimed to achieve tumour-free margins. To reach this goal, all requirements related to the treatment of invasive cancer are applicable to DCIS, in particular:
Optimal imaging, including magnification views in cases of microcalcifications
Preoperative diagnosis of microcalcifications or density by histological core needle biopsy stereotaxis or ultrasound guided
If necessary, discussion of the patient with the multidisciplinary team
Guide localisation preceding any surgery of a clinically occult lesion
Surgical resection targeted to at least 1 mm tumour-free margin
Specimen radiography after diagnostic and/or therapeutic excisional surgery
Marking of the specimen after excision to guide the pathologist
Diagnostic workup by the pathologist according to established guidelines
Complete excision of DCIS is the ultimate goal in management of DCIS. Surgery must also consider that the distribution of DCIS is usually segmental and grows toward the nipple and that biopsies should be done by the surgeon in the shape of a cone and toward the nipple [3]. Moreover, the potential propensity toward longitudinal rather than radial growth should be considered; therefore, glandular resection should be full-depth.
Although it is accepted that the duct system forms segments represented by the branching of a major lactiferous duct, there is no proof that each segment is encased in a fibrous or fascial capsule. For this reason, surgeons cannot identify a mammary segment during operation, and they are obliged to focus their attention on removal of the areas of mammographically detected density or microcalcification. Nonetheless, attempts can and should be made to remove the lesion in a more or less conical shape along the segmental route of possible extension toward the nipple (Fig. 12.2). The resected specimen should have sutures identifying the direction of the nipple and the superior, anterior, and lateral margins. Regardless of their precise shape, these samples should be serially sectioned toward the direction of the nipple to evaluate the extent of the lesion’s spread from the TDLU(s) of origin.
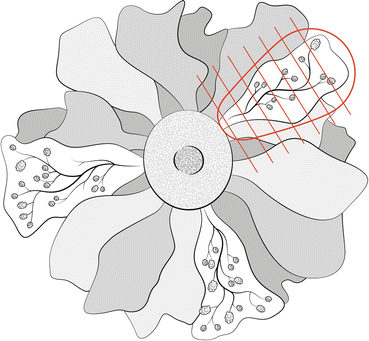

Fig. 12.2
Graphic representation of the segmental distribution of mammary duct system, optimal conical excision of a segment involved by DCIS with the tip of the cone pointing toward the nipple, and optimal orientation and serial sectioning of the excised sample from the periphery toward the nipple (Adapted from Tavassoli [3]). This drawing also depicts as ductal-lobular segmental units radiate from the nipple in an unevenly and sometimes unpredictable arrangement
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








