Types
Incidence (%)
Mean age range
Prognosis
Non special type
50–75
Intermediate
Lobular
5–15
63
Better than NST
Tubular
1–2.5
58–64 years
Good
Mucinous
1–5
59–71 years
Very good
Cribriform
0.5–3
53–58 years
Excellent
Medullary
1
52 years
Variable
Micropapillary
1.2–2.3
53–59 years
Similar to NST
Metaplastic
1
50–60 years
Worse
Apocrine
0.5–3
Similar to NST
Classification of an invasive BC as special type is based on the finding of the typical morphological features of that special type in more than 90 % of the neoplasia. When a BC shows two different morphological aspects and none of these histological aspect is represented by more than 90 %, the tumour is classified as mixed. The most frequent mixed types of invasive BCs are represented by a combination of NST carcinoma and lobular carcinoma, NST carcinoma and tubular/cribriform carcinoma, or NST carcinoma and mucinous carcinoma.
Microinvasive BC is a rare occurrence representing 0.5 % of invasive BC. Microinvasive BC is an invasive carcinoma with an extension of cancer cells beyond the basement membrane with no focus greater than 2 mm or with no more than 3 foci of invasion each <1 mm in its greatest dimension. The size of the individual foci should not be added together, but the size of the largest focus only is used to classify the microinvasion.
Microinvasive BC is the first stage in the development of invasion and can be seen in association with DCIS and LCIS. Conversely, most of these lesions upon review turn out to be T1a lesions with an extensive intraductal component. Comedo was reported to be the most common histological subtype of DCIS associated with microinvasion and often has a poor prognosis. Usually microinvasion is not associated with axillary LN invasion.
Coexisting lesions. Most BC develops in the TDLU, and often, associated pathologies (proliferating atypia or borderline lesions) can coexist with the main pathology. These coexisting pathologies are more often statistically determined than properly diagnosed. Moreover may be difficult to distinguish the multifocal forms of breast cancer from the multicentric ones and, just as most of the synchronous forms from the metachronous ones (see Sect. 15.1).
13.1.2 Main Types of BC
NST Carcinoma (Ductal Carcinoma NST). It frequency rises with increased patient age and is very uncommon before the age of 30 in patients without a family history of BC. On gross examination, NST carcinoma is a firm, well-defined to poorly defined, sometimes stellate nodule. Microscopically, malignant epithelial cells with different grades of atypia are arranged in tubules, trabeculae, or sheets. The nuclear atypia, the extension of tubular pattern, and the frequency of mitoses vary with the degree of differentiation.
Lobular Carcinoma (LIC). The mean patient age at presentation is 63 years. In recent years, its incidence seems to be increasing over that of other types of BC. Macroscopically, the appearance is variable, from a grey or white, firm, well-circumscribed mass to a not well-defined area of thickening. Histologically, lobular carcinoma may be subdivided in the following variants: classical, alveolar, solid, tubule-lobular, pleomorphic, and mixed.
The neoplastic cells are typically uniform and non-cohesive, with regular, round or oval, eccentrically placed nuclei with small nucleoli. The majority (75 %) of lobular carcinomas are classified as grade 2, 5 % as grade 1, and only 10 % as grade 3. LIC is immunohistochemically negative to E-cadherin in more than 85 % of cases.
The histological variant of invasive lobular carcinoma seems to be important for prognosis; the tubule-lobular variant has a very low risk of local and distant recurrence, whereas the solid variant has a high risk of regionally and distant-site recurrence (82 and 54 %, respectively). Metastatic pattern of LIC differs from that of invasive ductal carcinoma NST. LIC frequently metastasizes to bone, serosal cavities, gastrointestinal tract, uterus, ovary, and meninges, while invasive ductal carcinoma NST shows preferential tumour extension to the lung. Prognosis is a slightly improved than invasive ductal carcinoma NST.
Tubular Carcinoma. On gross examination, tubular carcinoma is a hard nodule with a stellate appearance usually ranging from 10 to 20 mm. Microscopically, it is entirely composed of angulated tubules with a single layer of epithelial cells often showing apical snouts. More than 90 % of the tumour must be composed of these tubules to classify as tubular carcinoma.
By definition, tubular carcinoma is of histological grade 1 as it scores 1 for tubule formation, 1 or rarely 2 for nuclear atypia, and 1 for number of mitoses. Even if nodal metastases can be detected in 12–19 % of the T1 cases, the prognosis is good.
Mucinous Carcinoma. On macroscopic examination it appears as a soft, well-circumscribed mass with a gelatinous aspect on a cut surface. The characteristic histological features are nests, trabeculae, acini, or sheets of neoplastic cells dispersed in a pool of extracellular mucin. Intracellular mucin may also be present with the presence of some signet-ring cells.
Mucinous carcinoma may have nodal metastases in 14 % of cases, and this principally depends on the size; for example, tumours less than 1 cm in size have less than 4 % risk of lymph node metastases. The prognosis is very good with an overall 5-year survival of 80–86 %.
Cribriform Carcinoma. At presentation, cribriform carcinoma has a mean size greater than 2.0 cm and on gross examination is a moderately well-defined mass with a stellate/grey cut surface. Microscopically, the neoplastic cells are organized in a cribriform pattern and have a low or, rarely, an intermediate cytonuclear grade. As with tubular carcinoma, these invasive tumours are often of histological grade 1. Lymph node metastases may be present in a percentage of cases similar to that reported for tubular carcinoma with only one or two metastatic nodes. The prognosis is excellent with a 5-year survival rate of almost 100 %.
Medullary Carcinoma. The average age of affected patients is 52 years, but half are less than 50 years old. Macroscopically, medullary carcinoma is a well-defined and well-circumscribed mass with a grey/tan cut surface; its average size is greater than 2.0 cm. This tumour is characteristically composed by pleomorphic nuclear grade 3 cells, arranged in a syncytial growth pattern for more than 75 % of the nodule, without glandular structures and with a diffuse, moderate to marked lymphoplasmacytic infiltrate which is present into and all around the tumour. To classify an invasive BC as a true medullary carcinoma, all the histological features cited above must be present. Prognosis is dependent on pathological findings due to the problematic reproducibility of its diagnosis. Nevertheless, it has been recorded that women with medullary node-negative carcinoma have a better prognosis than node-negative patients NST grade 3 (10-year survival rate of 84 % versus 63 %).
Micropapillary Invasive Carcinoma. Macroscopically it is a grey/white, stellate nodule with a mean size greater than 2.0 cm. Histologically, invasive micropapillary carcinoma is composed of nests of eosinophilic cuboidal/columnar cells surrounded by an artifactual clear space. This lesion is typically of histological grade 3 (58–82 %) or grade 2 (18–33 %) and shows lymphovascular invasion in the majority of cases (from 63 to 76 % in different series). Lymph node metastases have been recorded in 69–95 % of cases. Despite some discordant data, the prognosis of patients affected by invasive micropapillary carcinoma seems to be similar to that of patients affected by NST cancer when matched for other prognostic features.
Metaplastic Carcinoma. This neoplasia usually arises in the sixth and seventh decades of life as a palpable breast mass or, sometimes, as inflammatory carcinoma. On gross examination, metaplastic carcinoma is a solid mass greater than 3.0 cm, which typically has a tan/white cut surface; cystic areas may be present. Microscopically, metaplastic carcinoma is composed of spindle cells in about 70 % of cases; the cells show moderate/severe nuclear atypia with a conspicuous number of mitoses and are arranged in fascicles, possibly with a storiform pattern. Many times a squamous differentiation and/or an association with intraductal carcinoma or NST invasive carcinoma is present. Metaplastic carcinomas are generally of histological grade 3, but the prognostic value of grading in metaplastic carcinoma is uncertain. This type of tumour is typically negative for ER, PR, and HER2. Lymph node metastases are less frequent in metaplastic cancers than in invasive carcinoma NST of similar size and grade. However, as in other triple-negative BCs, distant metastases, preferentially brain and/or lung metastases, can be found at the time of diagnosis. Metaplastic BCs have lower response rates to conventional adjuvant chemotherapy and a worse clinical outcome than those of other types of triple-negative BCs.
Apocrine Carcinoma. This tumour has clinical characteristics similar to those of NST invasive carcinoma. Also on gross examination, apocrine carcinoma lacks specific features. Microscopically, the neoplastic cells show typical apocrine differentiation with abundant eosinophilic granular cytoplasm and large nuclei with prominent nucleoli. Many studies have shown no difference in outcome, including survival, between apocrine carcinomas and NST invasive cancers when matched for standard prognostic parameters.
13.1.3 Location of Breast Cancer
Clinically, the breast is divided into five quadrants (upper outer, lower outer, upper inner, lower inner, and central). This arbitrary distinction may be impractical for big tumours, which occupied more than one quadrant, or small tumours located on the interface between two quadrants, e.g. at 12, 3, 6, and 9 o’clock, should be classified in a separate category. The approximate percentage of all BC found in each quadrant is represented in Fig. 13.1.
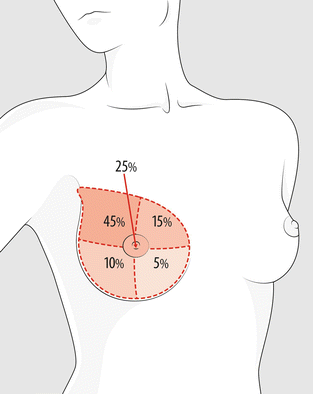

Fig. 13.1
Clinical quadrants of the breast with the approximate percentage of all BC found in each
From a practical point of view, this spatial definition was based on the assumption that unicentric cancers arising in one quadrant of the breast were more likely to grow from the same ductal structures, while multicentric BC were more likely to occur in separate areas of the breast. The application of molecular biology techniques to this problem has provided some clarification of these issues. Molecular studies suggest that most, but not all, multicentric carcinomas arise from the same cells so that the terms multicentric and unicentric-multifocal do not indicate two biologically distinct processes. In general, the term multifocality indicates lesions in closer proximity than those designated as multicentric, but the therapeutic considerations for both groups are similar (see Sect. 15.1).
However, definitions of multicentric and unicentric-multifocal are problematic since the ‘quadrants’ of the breast are arbitrary external designations, whereas no internal boundaries exist. In addition, these definitions may result in similar lesions being classified differently, based on their location in the breast, irrespective of its size. For example, two lesions at 11 and 1 o’clock could be classified as multicentric because they occur in the upper outer and upper inner quadrants, whereas two lesions could be classified as multifocal if they are at 1 and 3 o’clock.
Finally, some considers the location of the tumour within the breast an important predictor. Tumours that develop toward the outside of the breast tend to be less difficult to treat than those that occur more toward the middle of the breast.
13.1.4 Staging of Breast Cancer
TNM system. TNM is the most widely used system for BC staging [2]. It works in a two-step procedure which first classifies cancer by several factors (T for tumour, N for nodes, M for metastasis) and then groups these TNM factors into overall stages. It is crucial to be aware that the TNM system criteria have varied over time, sometimes fairly substantially, so that reports often use the staging edition that was in place when the study began rather than the date of acceptance or publication. The AJCC Seventh Edition TNM Classification for Breast Cancer is presented in Table 13.2.
Primary tumour (T) | |
TX | Primary tumour cannot be assessed |
T0 | No evidence of primary tumour |
Tis | Carcinoma in situ |
Tis (DCIS) | Ductal carcinoma in situ |
Tis (LCIS) | Lobular carcinoma in situ |
Tis (Paget) | Paget disease of the nipple not associated with invasive carcinoma and/or carcinoma in situ (DCIS and/or LCIS) in the underlying breast parenchyma. Carcinomas in the breast parenchyma associated with Paget disease are categorized based on the size and characteristics of the parenchymal disease, although the presence of Paget disease should still be noted |
T1 | Tumour ≤20 mm in greatest dimension |
T1mi | Tumour ≤1 mm in greatest dimension |
T1a | Tumour >1 mm but ≤5 mm in greatest dimension |
T1b | Tumour >5 mm but ≤10 mm in greatest dimension |
T1c | Tumour >10 mm but ≤20 mm in greatest dimension |
T2 | Tumour >20 mm but ≤50 mm in greatest dimension |
T3 | Tumour >50 mm in greatest dimension |
T4 | Tumour of any size with direct extension to the chest wall and/or to the skin (ulceration or skin nodules) |
T4a | Extension to chest wall, not including only pectoralis muscle adherence/invasion |
T4b | Ulceration and/or ipsilateral satellite nodules and/or oedema (including peau d’orange) of the skin, which do not meet the criteria for inflammatory carcinoma |
T4c | Both T4a and T4b |
T4d | Inflammatory carcinoma |
Regional lymph nodes (N) | |
Clinical | |
NX | Regional lymph nodes cannot be assessed (e.g. previously removed) |
N0 | No regional lymph node metastasis |
N1 | Metastasis to mobile ipsilateral level I, II axillary lymph node(s) |
N2 | Metastases in ipsilateral level I, II axillary lymph nodes that are clinically fixed or matted or in clinically detecteda ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastasis |
N2a | Metastases in ipsilateral level I, II axillary lymph nodes fixed to one another (matted) or to other structures |
N2b | Metastases only in clinically detecteda ipsilateral internal mammary nodes and in the absence of clinically evident level I, II axillary lymph node metastases |
N3 | Metastases in ipsilateral infraclavicular (level III axillary) lymph node(s), with or without level I, II axillary node involvement, or in clinically detecteda ipsilateral internal mammary lymph node(s) and in the presence of clinically evident level I, II axillary lymph node metastasis; or metastasis in ipsilateral supraclavicular lymph node(s), with or without axillary or internal mammary lymph node involvement |
N3a | Metastasis in ipsilateral infraclavicular lymph node(s) |
N3b | Metastasis in ipsilateral internal mammary lymph node(s) and axillary lymph node(s) |
N3c | Metastasis in ipsilateral supraclavicular lymph node(s) |
Pathologic (pN) b | |
pNX | Regional lymph nodes cannot be assessed (e.g. previously removed or not removed for pathologic study) |
pN0 | No regional lymph node metastasis identified histologically. Note: isolated tumour cell clusters (ITCs) are defined as small clusters of cells ≤0.2 mm, or single tumour cells, or a cluster of <200 cells in a single histological cross section; ITCs may be detected by routine histology or by immunohistochemical (IHC) methods; nodes containing only ITCs are excluded from the total positive node count for purposes of N classification but should be included in the total number of nodes evaluated |
pN0(i−) | No regional lymph node metastases histologically, negative IHC |
pN0(i+) | Malignant cells in regional lymph node(s) ≤0.2 mm (detected by hematoxylin-eosin [H&E] stain or IHC, including ITC) |
pN0(mol−) | No regional lymph node metastases histologically, negative molecular findings (reverse transcriptase polymerase chain reaction [RT-PCR]) |
pN0(mol+) | Positive molecular findings (RT-PCR) but no regional lymph node metastases detected by histology or IHC |
pN1 | Micrometastases or metastases in 1–3 axillary lymph nodes and/or in internal mammary nodes, with metastases detected by sentinel lymph node biopsy but not clinically detectedc |
pN1mi | Micrometastases (>0.2 mm and/or >200 cells, but none >2.0 mm) |
pN1a | Metastases in 1–3 axillary lymph nodes (at least 1 metastasis >2.0 mm) |
pN1b | Metastases in internal mammary nodes, with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detectedc |
pN1c | Metastases in 1–3 axillary lymph nodes and in internal mammary lymph nodes, with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected† |
pN2 | Metastases in 4–9 axillary lymph nodes or in clinically detectedd internal mammary lymph nodes in the absence of axillary lymph node metastases |
pN2a | Metastases in 4–9 axillary lymph nodes (at least 1 tumour deposit >2.0 mm) |
pN2b | Metastases in clinically detectedc internal mammary lymph nodes in the absence of axillary lymph node metastases |
pN3 | Metastases in ≥10 axillary lymph nodes; or in infraclavicular (level III axillary) lymph nodes; or in clinically detectedd
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|