Fig. 14.1
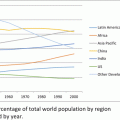
Over the past decade, the number of clinical investigators based in the United States has remained relatively flat (gray arrow), while the number engaged in research outside the United States has nearly tripled (black arrow). There has been a gradual trend of shifting investigators and investigative sites overseas. The number of clinical trials investigators abroad has been growing at a rate of 5–15 %, depending upon the country. In the United States, there has been a slow decline in the number of active clinical investigators at a rate of 1–3 % per year. Some of the attrition is from retirement of existing investigators, and the remainder is from insufficient recruitment and early abandonment of clinical research
14.5.6 More Potential Subjects
More patients are needed to do clinical trials. In the United States, overall participation rate is 1–2 %, and estimates from 2005 were that 20 million subjects were needed to participate in all ongoing clinical studies. Overseas clinical trial supercenters can see many patients (some see 9,000 outpatients/day), and some specialize in certain diseases, such as psoriasis or atopic dermatitis. These types of centers do not exist in the United States.
14.5.7 Disease Variability
Diseases may also manifest differently overseas, and responses to therapy may vary by ethnic group. For example, Iressa is effective in Japanese patients, but not in US patients. There are also different dermatologic concerns in global markets. For example, pigmentary disorders and post-inflammatory pigmentation feature prominently as a concern among patients in Latin America, the Middle East, the Indian Subcontinent, Africa, and Asia but are less critical in Western Europe, Canada, and the United States.
Countries have different levels of attractiveness based on patient pool, cost efficiency, regulatory climate, expertise, and infrastructure. This was studied by A.T. Kearney (Bailey et al. Make Your Move: Taking Clinical Trials to the Best Location, Pharmafocusasia.com). They ranked China a close second, however, recently, it has fallen into disrepute for data fabrication and bribery.
14.5.8 Push and Pull
In summary, overseas growth is occurring because of a push and a pull. Regulations and other hindrances are pushing sponsors to do studies outside United States. Lower costs and eager investigators and plentiful study subjects are pulling sponsors abroad. A breakdown of the drivers of overseas growth follows:
Access to patients.
In Asia, Wyeth has identified Phase II Super Centers (9,000 outpatient visits/day; Patrick McGee “Clinical Trials on the Move”).
There are overseas hospitals specializing in diseases (heart disease, diabetes, etc.).
Many overseas patients are treatment naïve.
Efforts are needed to test medications in different ethnic groups (Iressa is effective in Asians, but not in the United States).
Approved doses vary by country (Japanese doses tend to be lower in 1/3 of cases).
ADRs differ among ethnic groups.
Slow recruitment causes 85–90 % of the delay and is costly.
Recruitment rates are higher, and costs are cheaper abroad.
Local clinical trials also groom markets and grease wheels in developing countries seeking status and recognition.
14.5.9 Pitfalls
Some US plans prohibit participation in clinical trials. HIPAA makes recruitment difficult. The basic standard of care in the United States is high which competes with clinical trials. In the third world, participation in a clinical trial may lead to better care. However, as the uninsured population increases, this trend may not continue. Migration of clinical trials is not a panacea for industry. Drawbacks to overseas growth include:
Little information on foreign IRBs.
Difficulty in auditing foreign PIs.
Patient understanding and education may vary.
This calls into question adequacy of informed consent and level of volunteerism in participating.
Lack of a skilled workforce. There is a shortage of mentors, of qualified academic staff, inadequate academic infrastructure, and a flight of well-educated well-qualified workers to developed countries. The number of physicians in Rwanda per 1,000 is 0.02, while that number is 3.58 in Sweden.
Trend away from medical school. Applications to medical school in developing countries are down over the past 20 years.
Lack of infrastructure. Whether referring to teaching and research opportunities or civil unrest or unpredictable electricity or inadequate roads, many factors conspire against a stable clinical research workforce.
Diversion. Studies sponsored by multinational corporations drain already thin resources and recruit what few clinicians and scientists are in country away from pressing social needs to high status jobs in gleaming privately funded laboratories.
Respect. Investigators in developing nations complain that their work is not recognized by grant agencies or scientific journals and is often rejected or neglected. This erodes morale.
Cost. Maintaining and supplying a modern laboratory in a country with limited transport, limited clean water, inadequate sewage, and inadequate power requires costly importation and repair. Often, if equipment breaks down, it is not repaired because shipping parts from overseas becomes too cumbersome.
Strife. Whether political unrest, war, or natural calamity, resources from health care are often stretched thin, treating war casualties, victims of epidemics or famine, or victims of natural disasters.
Attitudes. Cultural and social beliefs combined with varying levels of basic education may alter participation rates or approval mechanisms for clinical trials. There may also be suspicions of caregivers of a different ethnic background than the population being studied.
Ethics. Poor populations in developing countries are particularly vulnerable to coercion or inducement. The ethical requirements of IRBs vary among nations.
14.5.10 Cost Savings
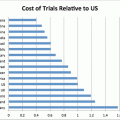
Many US companies are shifting trials overseas because they are cheaper, and pools of subjects are larger. Issues raised include uniformity of standards and regulatory issues. Race, justice, and economics also come into play. Vulnerable populations overseas share the following characteristics:
Limited economic development.
Inadequate protection of human rights.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree