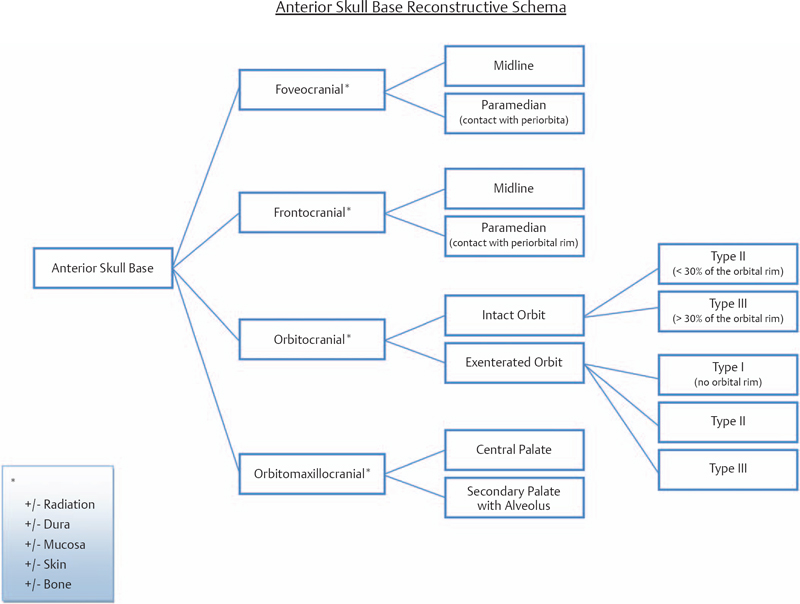
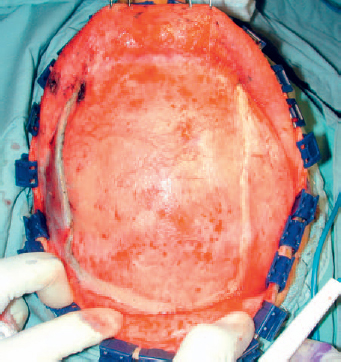
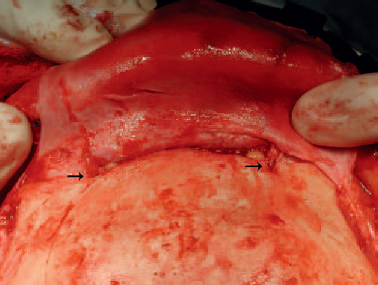
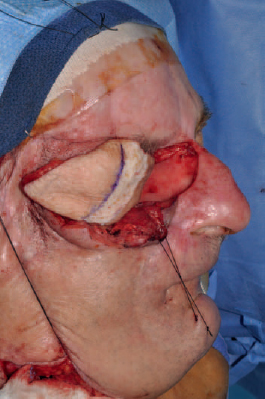
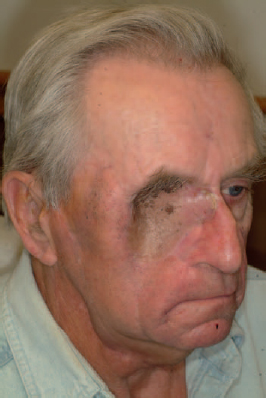
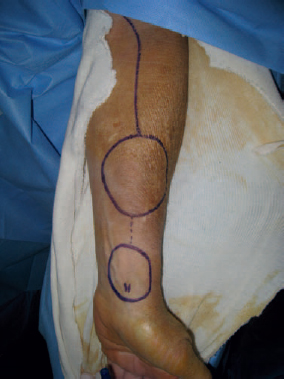
6 Advances in reconstructive techniques have enabled improved functional outcomes after ablative skull base surgery. Reconstruction of cranial base defects involves separating the sterile cranial cavity from a contaminated upper aerodigestive tract, middle ear/mastoid, and orbits. Paramount to this task is obtaining a watertight seal of the dura to prevent potential intracranial complications such as meningitis and intracranial abscess. Perioperative radiotherapy can compromise wound healing, lead to resorption of nonvascularized tissues, and predispose patients to wound infections. In the setting of benign disease or when perioperative radiotherapy is not necessary, reconstruction may be more straightforward often requiring only local flaps or in some cases nonvascularized tissue. However, the addition of radiation provides additional considerations in reconstructive decision making. The concept of a reconstructive ladder, utilizing local, regional, or free tissue for the increasing extent of defect, is a model that may not apply in skull base defects, because the best reconstructive option is the one that will be most effective in preventing complications. Neligan et al1 showed that free flaps have substantially fewer wound complications than local and regional flaps (10% versus 20.4% and 36.3%, respectively). Similarly, free flaps are associated with lower rates of cerebrospinal fluid (CSF) leaks, meningitis, and abscess. Pedicled myocutaneous flaps such as the pectoralis major flap often cannot reach the skull base and may suffer from distal necrosis, resulting in the aforementioned complications. Smaller defects may require free tissue transfer in the setting of radiation, in the case of revision surgeries, and when dead space must be obliterated. More recent advances in the endoscopic approach to skull base extirpation have been paired with novel techniques in endoscopic reconstruction and may lead to a reconstructive approach from the nasal cavity as opposed to from the cranial cavity. The reconstructive surgeon may take advantage of a different armamentarium of reconstructive options for this approach. Regardless of whether an open or endoscopic approach to reconstruction is utilized, the principles are still the same: to create a stable barrier to separate a sterile cranium from a contaminated upper aerodigestive tract. The skull base is composed of five bones: the frontal, ethmoid, sphenoid, occipital, and parietal. It is further divided into regions that house different components of the brain known as the anterior, middle, and posterior cranial fossae. The anterior cranial fossa is bounded anteriorly by the frontal bones and posteriorly by the anterior clinoid processes and the planum sphenoidale. The crista galli, a vertical projection of the ethmoid bone, sits in the midline. The horizontal component of the ethmoid bone is composed of the cribriform plate through which the olfactory filaments descend into the nasal cavity and the fovea ethmoidalis. Laterally the frontal bones compose the orbital roof. Communications between the cranium and the sinonasal cavity such as the foramen cecum and the olfactory foramina may provide a potential route for the spread of infection. The middle cranial fossa is bounded anteriorly by the greater wing of the sphenoid, which also forms the anterior floor. The petrous temporal bone makes up the posterior floor of the middle fossa. Centrally the greater wing of the sphenoid makes the sella turcica bounded anteriorly by the anterior clinoid processes and posteriorly by the posterior clinoid processes. The floor of the middle cranial fossa has numerous foramina through which cranial nerves exit the cranium. The posterior cranial fossa is composed of the sphenoid, occipital, and temporal bones. Anterior to the foramen magnum, the sphenoid and occipital bones form the bony clivus. Posteriorly, the occipital bone medially meets the posterior petrous temporal bone to make up the floor of the posterior fossa. The ideal classification system is one that would categorize defects according to reconstructive options. Such a classification system has not yet been developed. The difficulty in developing this classification system relates to the anatomic complexity of the skull base, the variability of the defects, and the need to strike a balance between making the system simple enough for effective communication by clinicians but complex enough to be useful as a defect-based decisionmaking tool. Jackson and Hide2 initially described a classification of skull base lesions that has largely been replaced by a schema introduced by Jones et al3 in which anterior, middle, and posterior regions correlate to the anterior, middle, and posterior cranial fossae. Irish and colleagues4 reviewed 77 patients with skull base neoplasms and further classified tumors into skull base regions (I, II, and III) based on anatomic boundaries and tumor growth patterns (Fig. 6.1). Region I tumors arise from the sinuses, orbit, and nasal cavity and extend to involve the anterior cranial fossa. Region I tumors also include those that arise from the clivus and extend posteriorly to the foramen magnum. Region II tumors originate in the lateral skull base and extend into the infratemporal and pterygopalatine fossa with involvement of the middle cranial fossa. Region III lesions arise from the ear or parotid or temporal bone and extend intracranially to involve the posterior cranial fossa. This classification is useful for describing the specific area of the skull base that requires reconstruction.5–8 Fig. 6.1 Irish classification. The skull base has been described by Irish et al4 to be divided into three regions based on the anatomic location and growth pattern of skull base tumors. Region I involves the anterior skull base. Region II tumors originate in the lateral skull base and extend into the infratemporal and pterygopalatine fossa with involvement of the middle cranial fossa. Region III lesions arise from the ear, parotid, or temporal bone and extend intracranially to involve the posterior cranial fossa. Table 6.1 The Mount Sinai Classification of Skull Base Defects (Modified) Primary repair Patch graft II Mucosa Nasal-nasopharyngeal Oro-oropharyngeal Sphenoid III Skin Scalp Forehead Midface Lower face Neck Auricle IV Bone Calvaria Zygoma Palate Mandible Orbital floor Temporal V Cavitites Cranial Sinonasal Orbital Oral Note: In this classification system, skull base defects are defined according to elements that have an impact on morbidity and functional outcome to compare the reconstruction of defects of similar extent. The five types of defects that are most commonly reconstructed are shown. More recently, the Memorial Sloan-Kettering group described a classification system that includes defects of the anterior and middle cranial base.9 The anterior base defects are categorized as a simple or complex resection. Simple defects include the skull base at the cribriform plate adjacent to a tumor, which may include removal of a portion of the palate or the orbital contents. Complex defects include the floor of the anterior cranial fossa adjacent to the lesion, dura, or brain, with or without orbital contents, and the nasal cavity, maxilla, and the palate. The most sophisticated description of skull base defects was developed by the Mount Sinai group (Table 6.1).10 This classification system takes into account the individual anatomic elements that are involved in skull base defects. It includes seven major defect categories: (1) dura, (2) bone, (3) cutaneous, (4) mucosal, (5) cavity, (6) neurologic, and (7) carotid artery. These defect categories serve as a framework to guide the reconstructive surgeon to avoid potential morbidity. Dural defects closed primarily are at lower risk than those that require a patch graft after dural resection. Similarly, more extensive mucosal defects may predispose one to the introduction of secretions that may contaminate the CSF. Cranial nerve deficits may affect both functional and cosmetic outcomes. The classification system is very useful for providing a detailed description of the defect, giving detailed analysis for decision making, predicting potential postreconstructive complications, and in counseling patients. For general clinical use, we use a classification involving subdivision of the anterior cranial fossa defects into compartments that direct the reconstructive algorithm; these regions are foveocranial, frontocranial, orbitocranial, and orbitomaxillocranial (Fig. 6.2). Foveocranial defects are median or paramedian defects involving the ethmoid bone or the inner table of the frontal bone. Defects involving the orbit laterally or outer table of the anterior frontal bone do not qualify for this category of defects. Because the frontal and orbital rims remain intact, reconstruction of foveocranial defects often requires soft tissue coverage alone. Reconstruction of this area is focused on separation of the cranial compartment from the nasal cavity with a prerequisite watertight dural seal and use of vascularized tissue to prevent CSF leak and intracranial infectious complications. Before the use of local vascularized flaps, reconstruction of skull base defects consisted principally of split-thickness skin graft coverage. The importance of vascularized tissue was realized as Ketcham et al11 reported that approximately 50% of patients with skin grafts or tensor fascia lata grafts developed CSF leaks. Since that time, the use of pericranial and galeal flaps has shown superior results with regard to prevention of CSF leak and infection. Tumors in this location are well suited to endoscopic approaches, and innovations with local pedicled flaps have facilitated more reliable reconstruction. Prior to consideration of local, regional, or free flap coverage, one must ensure adequate dural closure. If the defect is small, a primary closure can be considered. With larger defects, Snyderman et al12 advocate the use of a sandwichtype approach for closure. A synthetic dural substitute can be used as an underlay graft between the dura and brain. The dura itself can be reconstructed with fascial grafts such as tensor fascia lata, synthetic acellular dermis, or in some cases pericardium. This then can be sutured to achieve a watertight closure or alternatively secured using U-clips—a deployable suture based on shape-memory metal. Although these techniques are relatively reliable in the nonradiated patient, the radiated patient represents a unique challenge. The compromise in tissue vascularity means that tissue healing is compromised. This is compounded in a previously operated field. Patients with a history of radiotherapy require special consideration and the liberal use of vascularized tissue whenever possible to enhance healing. Recent advances in transnasal expanded endoscopic approaches have allowed for better visualization and a larger scope of resection of skull base lesions. Reconstruction of larger defects previously required a separate external approach with its associated morbidity. Hadad and colleagues13 introduced a novel technique using a vascularized pedicled nasoseptal flap (Hadad-Bassagasteguy flap) that allows entirely endoscopic reconstruction of larger skull base defects. Hirsch14 in 1952 initially described a random septal flap for endoscopic repair of a CSF leak with the constraint of a broad base that limited rotation of the random flap. In contrast, the nasoseptal flap utilizes blood supply from the posterior nasoseptal artery. Multiple series have demonstrated success with the nasoseptal flap for repair of CSF leaks and reconstruction of skull base defects after extended endoscopic approaches.13,15,16 The superiority of the nasoseptal flap over septal flaps performed in previous decades is attributed to its superior arc of rotation and large surface area. The risk of postoperative CSF leak from skull base ablative procedures reconstructed with the nasoseptal flap has decreased to approximately 5%, comparable with that of open reconstructive techniques.15 More recently, the nasoseptal flap has been utilized in a “reuse” manner for cranial base defects and adds to its versatility as a reconstructive option for expanded endoscopic resection of skull base lesions. This “takedown technique” for patients who require additional ablative surgery or for planned staged surgery has reasonably good success.17 In the case of tumor recurrences one should exercise caution in using the “takedown” technique. ♦ The blood supply to the nasoseptal flap is the posterior septal branch of the sphenopalatine artery. ♦ Prior to the procedure, adequate exposure can be achieved by decongesting the nose with topical adrenaline, and often resection of the middle turbinate is necessary to provide access to the tumor and for the reconstruction. ♦ A needle-tip monopolar cautery or contact laser is used for the mucoperichondrial incisions starting with the posteroinferior incision along the choana and extending along the inferior septum. The posterosuperior incision is placed through the natural sphenoid os and extends superiorly (1 cm below the cranial base to avoid damage to olfactory mucosa), whereas the inferior incision travels along the maxillary crest aspect of the septum. A wider flap may be harvested by extending the inferior septal incision to include the mucoperiosteum of the nasal floor. The two septal incisions are then joined anteriorly by a vertical incision. ♦ Prior to elevation of the flap, detaching the fibers at the front wall of the sphenoid and rostrum with a Cottle elevator can ease flap elevation. ♦ A submucoperichondrial plane is used for the dissection. ♦ The flap can be stored in the nasopharynx or in an enlarged natural maxillary sinus ostium for the duration of the procedure to avoid interfering with tumor resection. Careful consideration should be given to patients with tumors involving the septal mucosa. The nasoseptal flap is typically raised at the onset of the procedure, and one should not compromise oncologic principles to use this flap. A careful plan of the ablative cuts should be determined prior to raising the flap if the tumor is in proximity of the flap. Perioperatively, the reconstruction is often bolstered by either nasal packing or a Foley catheter inflated with sterile water. The Foley catheter can be deflated between day 1 and day 7, depending on the extent of the resection and the surgeons concern of stability of the reconstruction. The denuded nasal septum is left to granulate and can typically take roughly 3 months to remucosalize.18 Rigorous follow-up by the endoscopic surgeon for debridement as well as aggressive nasal hygiene using saline rinses will facilitate return to normal nasal function. The subgaleal fascia is deep to the galea. When the subgalea is raised with the periosteum of the skull, it is termed the pericranial flap. The use of a pericranial flap was first reported by Wolfe19 and expanded by Johns et al20 in a series of four patients with craniofacial defects. This dense vascularized connective tissue was found to be a practical reconstructive option given the access already employed in the bicoronal approach for ablative skull base surgery. In initial applications of the pericranial flap, skin grafts were used to line the nasal side of the flap. Snyderman et al21 subsequently reported that low complication rates were achieved without skin grafts in a review of 30 patients whose anterior cranial base defects were reconstructed with pericranial flaps alone. The pericranial flap has proven to be very reliable when used in limited anterior skull base reconstruction with minimal donor-site morbidity. The risk of CSF leak after reconstruction using this flap has been consistently reported to be approximately 5%. The flap is thin, pliable, and reported to be well vascularized even after regional irradiation. If the pericranial flap is available, particularly with open approaches, it is the best option for supporting a dural closure and separating the cranial and nasal cavities in foveocranial defects. Some authors have proposed that a more robust blood supply can be obtained if the galeopericranial flap is elevated.22 Including the galea may only marginally increase the distal blood supply of the pericranial tissue, and dissection of galea from the overlying skin can result in cutaneous necrosis. For these reasons the galea is rarely incorporated because readily available, unradiated, autogenous tissue can be transplanted into the defect. ♦ The pericranial flap is based on the supraorbital and supratrochlear arteries (Fig. 6.3). ♦ Harvest of the pericranial flap involves elevation in the subgaleal plane down to the level of the supraorbital rims. In the supraorbital region, the supraorbital notch is identified and the supraorbital vascular bundle is freed (Fig. 6.4). ♦ If a foramen is present, a 4-mm osteotome can be used to preserve the neurovascular bundle and maintain the arterial supply to the flap. ♦ The periosteum is then transversely incised 10 to 15 cm superior to the supraorbital rims and the flap elevated inferiorly beyond the supraorbital rim. It is protected anteriorly while osteotomies and tumor extirpation are performed (Fig. 6.5). ♦ At the time of reconstruction, the pericranial flap is placed intracranially after the supraorbital bone is re-secured to allow for coverage of the frontal bar in anticipation of adjuvant radiation (Fig. 6.6). ♦ In cases of revision surgery or frontal lobe atrophy, the volume between the frontal dura and cranial side of the frontal bone becomes great enough that one cannot be confident that the reconstruction will heal without infection or necrosis. In these circumstances, autogenous tissue transplantation is indicated. ♦ For endoscopic cases, the pericranial flap can be elevated through a minimally invasive approach using two small scalp incisions measuring 2 cm and 1 cm, and one transverse glabellar incision measuring 1 cm. The flap can be raised endoscopically with an elevator through one incision and an endoscope through the second scalp incision. Through the glabellar incision, a nasal through-and-through osteotomy can be created using a cutting burr to transpose the flap intranasally.23 Further investigation is still required to understand the overall effectiveness and utility of this approach. The pericranial flap is ideal for large foveocranial defects that require vascularized coverage of the defect. Its use can also be extended to provide vascularized tissue to surround free grafts, as will be discussed later in this chapter. The minimally invasive pericranial flap is ideal for patients who undergo endoscopic procedures in whom a nasoseptal flap is precluded for oncologic reasons. Perioperative care involves appropriate wound care for the bicoronal incision and other nasal hygiene measures applicable to the nasoseptal flap. The temporoparietal fascia flap (TPFF) has been utilized extensively in head and neck reconstruction. Fortes et al24 describe a technique in which the TPFF is delivered into the nasal cavity through a temporal-infratemporal soft tissue tunnel and transpterygoid window. This approach was reported in two patients without postoperative CSF leak and without complication. Harvested from the lateral parietal skull, the flap has a rich vascular supply based on the superficial temporal vessels. ♦ The temporoparietal fascial flap is supplied by the superficial temporal artery and vein. ♦ A coronal incision is made with care in the preauricular region to avoid injuring the vascular pedicle as it travels superficially anterior to the helical root. ♦ The anterior fascial incision must be posterior to the frontal branch of the facial nerve to avoid paralysis. ♦ To communicate the flap intranasally, an endoscopic transpterygoid approach is needed to open the contents of the pterygopalatine fossa. The external flap is communicated internally by first releasing the temporalis from the lateral orbital wall through a lateral canthus incision. Tracheal dilators are then used to open a space and transpose the flap intranasally.24 In previously operated patients with a healed coronal incision, the vascular pedicle may be compromised, precluding the use of this flap. This flap may be used more frequently in endoscopic sinus surgery cases as an alternative to the nasoseptal flap in cases where this flap is unavailable due to previous use or tumor involvement. When a nasoseptal flap is unavailable, a palatal island mucoperiosteal flap may be transposed into the nasal cavity through limited enlargement of a single greater palatine foramen. This flap was popularized by Gullane and Arena25 for palatal reconstruction. Oliver et al26 have demonstrated the use of this flap in skull base reconstruction for defects of the planum, sella, and clivus. The feasibility of such a flap was shown through cadaver dissections, but further evidence is needed to prove its effectiveness in reconstruction. ♦ The blood supply to the palatal island flap is the greater palatine artery and vein. ♦ Design of the flap should ensure adequate coverage of the skull base defect. Oliver et al26 suggest as much as 12 to 18 cm2 defects can be covered by this flap. ♦ The pedicle can be as long as 3 cm, but mobilization of the flap can be facilitated by opening the palatine foramen using a small osteotome. ♦ Dissection of the flap is in the submucoperiosteal plane. ♦ Careful orientation of the flap should avoid twisting of the vascular pedicle. ♦ Delivery to the skull base is performed by enlarging the greater palatine foramen and delivery of the flap. Like the temporoparietal flap, this flap can be used in instances when the nasoseptal flap is unavailable for use. In the immediate postoperative setting, patients can be started on a liquid diet for 2 days followed by a pureed diet for 2 days to allow for healing. Donor-site care involves oral hygiene with antiseptic mouth rinses. Remucosalization of the donor site occurs over the course of 4 to 6 weeks postoperatively. The FAMM flap has been described in cadaveric feasibility studies but has yet to be employed in clinical practice. The Pittsburgh group described the use of this flap in skull base reconstruction.27 Described as the facial buccinator flap, this musculomucosal flap can be rotated 180 degrees and delivered into the nasal cavity through a maxillary window. It may have potential utility for reconstruction after expanded endoscopic approaches to skull base lesions, but further studies are required to prove its effectiveness in patients. The radial forearm fasciocutaneous free flap (RFFF) is an excellent option for low-volume reconstructions if local and regional tissues are not available. This donor site is reliable, has a long vascular pedicle, provides thin pliable tissue, and has been shown to be effective for closing CSF leaks.28,29 Despite its widespread application, the reports of its use in anterior skull base reconstruction are more limited but show it to be a favorable option. In a study of 10 patients with defects from anterior or lateral skull base lesions, reconstruction with the radial forearm flap led to no flap failures or infections and only one CSF leak.30 At the University of Michigan, Chepeha et al31 reported that 20 patients undergoing salvage surgery of the anterior skull base after previous surgery or radiation were reconstructed with radial forearm free tissue and had a low rate of CSF leak (5%) and low overall major complication rate (15%). Potential disadvantages of the radial forearm free flap over local flaps include a longer operative time and additional low risk of hand morbidity. Other fasciocutaneous or myogenous flaps may be utilized and designed to fit the foveocranial defect when the volume of the radial forearm donor site is not sufficient to fill the defect. ♦ The radial forearm flap is based on the radial artery and its venae comitantes (Fig. 6.9). ♦ An appropriate donor site should be selected in the nondominant hand of the patient and care should be taken in the preoperative setting to avoid intravenous cannulation of that arm. ♦ During the ablative approach, care should be taken to preserve the superficial temporal vessels for subsequent anastomosis. Where these vessels are not available, one should consider using the facial artery as the donor vessel with or without the use of vein grafting to achieve adequate pedicle length (Fig. 6.10). ♦ A template of the ablative defect should be created prior to harvesting the flap to ensure adequate tissue (Fig. 6.11). ♦ The flap pedicle can brought out anteriorly through the bifrontal or subcranial craniotomy and tunneled subcutaneously to reach the donor vessels. Care must be taken to ensure adequate room for the vascular pedicle and to avoid pressure on the pedicle after closure of the wound (Fig. 6.12). ♦ In cases where the frontal bone is resected for oncologic reasons, an osseocutaneous radial forearm free flap can be used for reconstruction (Fig. 6.13). Fig. 6.9 The orbital nasal defect. While the orbital nasal defect requires separation of the nasal and orbital cavities, minimal volume is required so that the orbital cavity can accommodate an orbital prosthesis at a later date. Fig. 6.11 A double skin paddle radial forearm flap can be used to partition the individual cavities. When a large area of vascularized soft tissue is needed for reconstruction, and pericranium is not available or provides inadequate coverage, this is a strong reconstructive option. In the postoperative setting, the arm should be splinted in the position of safety for a period of 7 days, and donor defects should be skin grafted when necessary. Occasional numbness to the dorsum of the hand can result from radial sensory nerve injury using this flap. Meticulous nasal hygiene should be instituted using saline irrigations and endoscopic debridement as necessary. Reconstruction of frontocranial defects requires either vascularized soft tissue of adequate bulk or a bony construct. A principal concern for frontocranial defects is sufficient obliteration of the potential dead space that may exist between dura and the frontal bone. If the frontal or scalp skin is missing, this surface must also be addressed. Soft tissue can obliterate space, but if muscle is used it will atrophy and decrease the volume of the reconstruction. The missing portions of the frontal bone—the floor, posterior table, anterior table, or brow—also direct the reconstructive algorithm. In general, a defect of the frontal brow greater than 4 cm should be reconstructed with vascularized bone in a patient who will undergo radiation or has already undergone radiation, unless significant patient comorbidities preclude the longer operative time. It has been demonstrated that patients with a history of prior skull base surgery or radiation have a lower risk of major postoperative complications related to salvage surgery when reconstructed with a vascularized autogenous transplant.31 The pericranial flap is the reconstruction of choice if local tissue is available and the patient does not have a history of radiation. In radiated patients, selective use of the pericranial flap is recommended for small defects. Defects can be reinforced using split calvarial bone. If this bone has been radiated, however, it is better to use a nonradiated donor site in the parietal region. Some authors show excellent results using pericranium to wrap nonvascularized bone grafts.32–34 In a series of 34 patients who were reconstructed with split calvarial bone graft wrapped in pericranium, 33 grafts survived.35 The authors concluded that defects measuring more than 3.0 cm ×4.0 cm should be reconstructed rigidly with bone grafts. The single graft failure was related to osteomyelitis and epidural abscess in a patient who had been treated with radiation. Sinha et al36 reported successful results in 20 patients with anterior skull base defects who were not radiated prior to surgery and were reconstructed with a “threelayer technique” in which the first layer is titanium mesh against dura followed by calvarial bone grafts; the two layers are then wrapped in a pericranial flap.36 Six of the patients were radiated postoperatively and none of the patients developed a CSF leak, infection, or exposure of grafts after at least 1 year of follow up. We suggest that these techniques are useful for radiated patients with bone defects less than 4 cm in length or 6 cm2. ♦ Split calvarial bone grafts can be harvested from the outer table of the cranium after raising a bicoronal skin flap and pericranial flap or by harvesting the inner table of a bone flap from a craniotomy. Grafts should be harvested in nonradiated areas to improve the chances of graft take (Fig. 6.14). ♦ For grafts from the outer table, a cutting burr is used to create an outline of the bone graft to be harvested. Care must be taken to avoid full-thickness cuts and dural injury (Fig. 6.15).
Skull Base Reconstruction
♦ RELEVANT ANATOMY
♦ CLASSIFICATION OF SKULL BASE DEFECTS
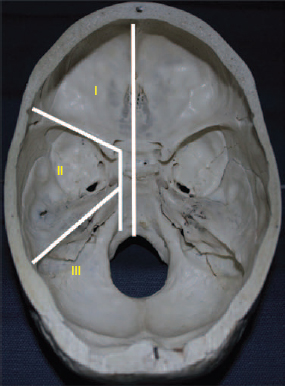
I
Dura
Intact
♦ FOVEOCRANIAL DEFECTS
Option for Management: Nasoseptal Flap
Surgical Technique and Considerations
Patient Selection and Perioperative Management
Option for Management: Pericranial Flap
Surgical Technique and Considerations
Patient Selection and Perioperative Management
Option for Management: Temporoparietal Fascia Flap
Surgical Techniques and Considerations (Figs. 6.7 and 6.8)
Patient Selection and Perioperative Considerations
Option for Management: Palatal Island Flap
Surgical Techniques and Management
Patient Selection and Perioperative Management
Option for Management: Facial Artery Mucosal Muscular (FAMM) Flap/Facial Buccinator Flap
Option for Management: Fasciocutaneous Free Tissue
Surgical Techniques and Considerations
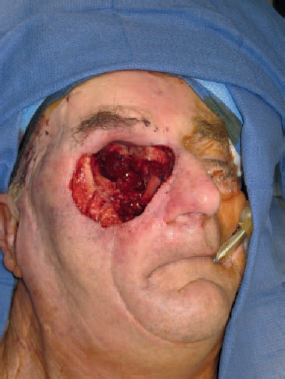
Patient Selection and Perioperative Management
♦ FRONTOCRANIAL DEFECTS
Option for Management: Pericranial Flap with Bone Graft
Surgical Technique and Considerations
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree