Scheme 17.1
A schematic representation of hexagonal phase
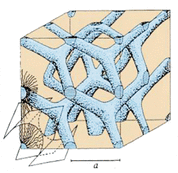
Whereas cubic liquid crystals are formed when the concentration of micelles dispersed in a solvent is sufficiently high that they are forced to pack into a structure having long-ranged positional order (Scheme 17.2).

Scheme 17.2
A schematic representation of cubic phase
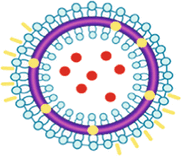
On the other hand, liposomes as lamella LC have an entrapped, discontinuous aqueous phase separated by bilayered lamellae from the continuous aqueous phase (Scheme 17.3).

Scheme 17.3
A schematic representation of a liposome
Thus, the evaluation of lamella LC as a drug carrier might be difficult because entrapped drug could easily transfer from the internal phase to the dispersion medium.
17.3 Current Problems in LC for TDDS
Phytantriol and glyceryl monooleate (GMO) are well-known compounds as non-lamella LC-forming lipoids (Phan et al. 2011). However, not all of drugs can be entrapped in non-lamella LC because hydrophilic/lipophilic balance (HLB) and molecular size of drugs (Charlotte and Drummond 2013) might affect the self-assemble of non-lamella LC-forming lipids. Furthermore, conventional non-lamella LC-forming lipids have so high viscosity that it would be tough to handle them in drug and cosmetic formulations and these lipids can form non-lamella LC in a narrow temperature range. These are problems that need to be overcome for the development of transdermal formulations using non-lamella LCs.
17.4 Structure of Liquid Crystal Dispersion
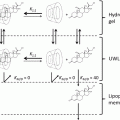
Figure 17.1 illustrates a scheme of the formation of lamella LC and non-lamella LC from amphiphiles in water (Gin et al. 2008a). Lamellar, hexagonal, bicontinuous cubic, and discontinuous cubic phases are well known and there are many research studies in that field. The structure of self-assembled mesophases is affected by amphiphile’s concentration. On increasing the concentration of amphiphiles in water, liposomes (lamellar), bicontinuous cubic lamella phases (Q2), and hexagonal phases (H2) are formed. There have been numerous descriptions of the liquid crystalline phase behavior (Hyde 1990). The dimensionless shape parameter known as critical packing parameter (CPP) has provided useful information regarding the choice of type of rational design of amphiphile-water phase behavior (e.g., micelle structure is when CPP <1, lamellar phase is when CPP=1, and inverse cubic structure is when CPP> 1) (Israelachvili 1976). In this chapter, we focus on cubic lamella phase and hexagonal phase that show skin permeation enhancement effects.
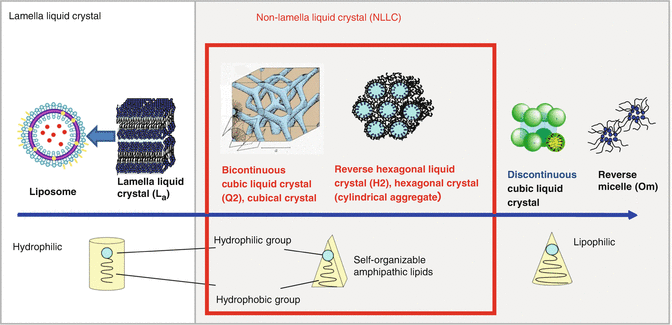

Fig. 17.1
Change of self-assembled structure of LC and non-lamella LC-forming lipids. The states of self-assembled structure depend on balance of polar/nonpolar groups (CPP value) and existence of salt or pH values in water phases. The arrow shows transitions of liquid crystals regarding lipophilicity of solvent
17.5 Preparation of a Mixture of Mono-, Di-, and Triesters (1) and Monoesters (2) Composed of Erythritol and Phytanylacetic Acid
Erythritol (2.50 kg) was dissolved in dimethyl sulfoxide (10.8 kg) at 100 °C under nitrogen purging, before the addition of anhydrous calcium carbonate (37.8 g) at 80 °C. Methyl phytanylacetate (5,9,13,17-tetramethyloctadecanoate) (4.83 kg) was added dropwise to the solution under reduced pressure over 2.5 h. The reaction mixture was refluxed under reduced pressure overnight, while the methanol produced was gradually distilled. After cooling, the mixture was neutralized by the addition of formic acid (29 g) and concentrated in vacuo. The residue (6.1 kg) was diluted with t-butyl methyl ether (18.3 kg) and filtered to remove the remaining erythritol. The filtrate was diluted again with t-butyl methyl ether (24 kg), washed twice with aqueous sodium bicarbonate, and concentrated in vacuo at 100 °C. The product obtained (4.7 kg, mixture 1) consisted of monoesters (36 %), diesters (12 %), and triesters (52 %) of erythritol and phytanylacetic acid (the ratio was determined by gas chromatography analysis). Mixture 1 was purified by column chromatography using silica gel (Wakogel C-300, Wako Pure Chemicals Industries, Ltd., Osaka, Japan) to afford monoesters 2 of 1-O- and 2-O-phytanylacetyl-erythritol.
17.6 Preparation of Liquid Crystal Dispersion
Table 17.1 shows the composition of liquid crystal A (LC-A) and liquid crystal B (LC-B) nano-dispersions. Ten grams of crude ester 1 or pure ester 2 was used to prepare LC-A and LC-B, respectively. In this step, LC-A and LC-B were semisolid and were nano-dispersed using a high-pressure emulsifier (NM2-L200AR, Yoshida Kikai Co., Ltd, Nagoya, Japan) or an ultrasonic homogenizer (USP-50, Shimadzu Corp., Kyoto, Japan), respectively, in aqueous solution (90.0 g) containing sodium calcein, Pluronic® F127 and methyl p-hydroxybenzoate. Calcein concentrations in LC-A and LC-B were different (see Table 17.1).
Table 17.1
Composition of liquid crystal dispersions
Ingredients | 30 mM calcein-entrapped LC-A (%) | Blank (%) |
1-o-(5,9,13,17-tetramethyloctadecanoyl)erythritol (crude, 1) | 10.0 | 10.0 |
Sodium calcein | 2.0 | – |
Pluronic® F127 (10 %) | 10.0 | 10.0 |
Methyl p-hydroxybenzoate | 0.1 | 0.1 |
Purified water | 77.9 | 79.9 |
Total | 100 | 100.0 |
Ingredients | 30 mM calcein-entrapped LC-B (%) | Blank (%) |
1-o-(5,9,13,17-tetramethyloctadecanoyl)erythritol (pure, 2) | 10.0 | 10.0 |
Calcein (sodium) | 0.2 | – |
Pluronic® F127 (20 %) | 10.0 | 10.0 |
Methyl p-hydroxybenzoate | 0.1 | 0.1 |
Purified water | 79.7 | 79.9 |
Total | 100 | 100.0 |
17.7 Structure of Liquid Crystal Dispersions Observed by Cryo-TEM Microscope
Skin permeation enhancement effects of non-lamella LCs, which consist of crude or pure non-lamella LC-forming lipids, such as 1-O-(5,9,13,17-tetramethyloctadecanoyl)erythritol (Fig. 17.2), are discussed in this chapter. The crude and pure esters were used to prepare non-lamella LC-A and non-lamella LC-B, respectively. Non-lamella LC-A- and non-lamella LC-B-forming lipids were dispersed in aqueous solution containing sodium calcein to prepare non-lamella LCs. Sodium calcein (M.W.; 623) is a good indicator used as hydrophilic fluorescent marker for skin permeation experiment. Thus, penetration-enhancing ability of non-lamella LC was investigated by evaluating the permeation of calcein through skin.
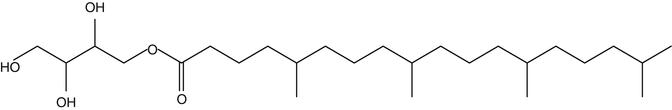

Fig. 17.2
Chemical structure of 1-o-(5, 9, 13, 17-tetramethyloctadecanoyl) erythritol
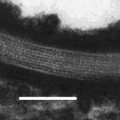
Figure 17.3 shows cryo-transmission electron microscope (TEM) micrographs of non-lamella LC-A and non-lamella LC-B. Similar to the cryo-TEM micrographs of cubic non-lamella LC prepared by monoolein and oleic acid (Garg et al. 2007; Gustafsson et al. 1996), non-lamella LC structures were observed in non-lamella LC-A and non-lamella LC-B (in white circles in Fig. 17.3). Figure 17.4 shows electronic diffraction patterns of non-lamella LC determined by cryo-TEM photographs. It was found from these diffraction patterns that non-lamella LC-A was a hexagonal non-lamella LC, having 4.6 nm periodic structure (A-2 dashed lines in Fig. 17.4), and non-lamella LC-B was a cubic non-lamella LC, having two periodic structures of 6.0 and 9.0 nm (B-2 dashed lines in Fig. 17.4).
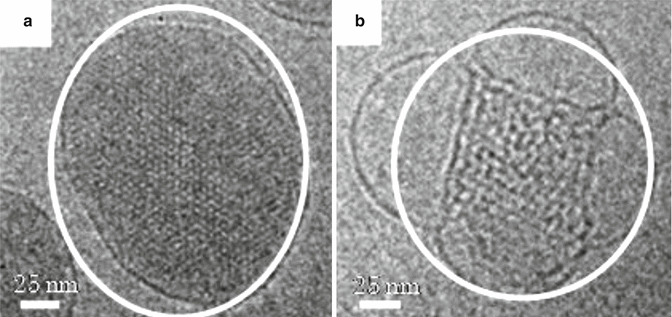
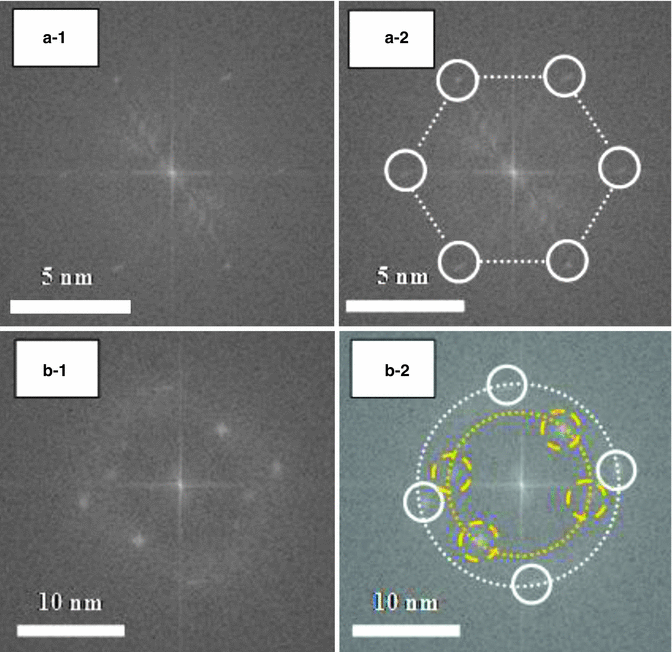

Fig. 17.3
Cryo-TEM microscope images of liquid crystal dispersions. Images (a, b) are non-lamella LC-A and non-lamella LC-B, respectively. Non-lamella LC structures were found in both (a, b) (see circles in images). Each white bar indicates 25 nm length

Fig. 17.4
Electron diffraction pattern of liquid crystal dispersions. (a-1) and (a-2) and (b-1) and (b-2) show the same images of LC-A and LC-B, respectively, without and with auxiliary lines and marks. (a-2) shows hexagonal LC, having 4.6 nm periodic structure. (b-2) illustrates cubic LC, having two periodic structures of 6.0 and 9.0 nm cycles. White bars indicate 5 or 10 nm length
17.8 Skin Penetration-Enhancing Effect of Liquid Crystals
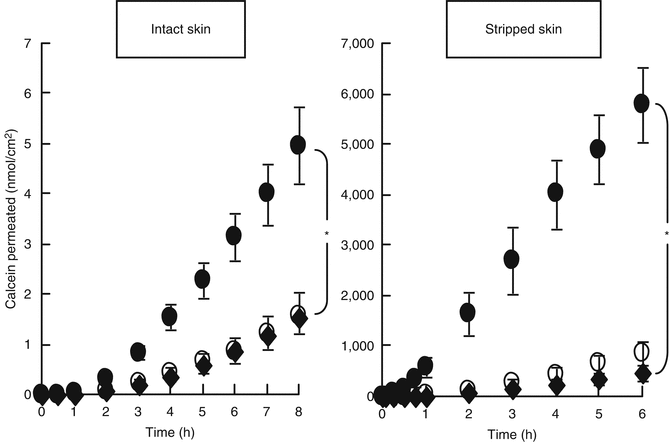
The penetration-enhancing effectiveness of non-lamella LC in topical formulations was evaluated by measuring permeation of a mal-absorbable compound, calcein, through excised hairless rat skin and through the three-dimensional cultured human skin model (LSE-high).
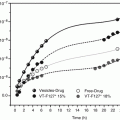
Figure 17.5 shows the time course of calcein permeation through excised hairless rat skin from the reverse-hexagonal liquid crystal (non-lamella LC-A) formulations. In both intact and stripped skin, the skin permeations of calcein from calcein entrapped in non-lamella LC-A formulation provided a 3 and 10 times higher, respectively, than the permeation of calcein from free calcein solution. In addition, the mixture of blank non-lamella LC-A dispersion and free calcein showed similar skin permeation to that of free calcein solution. Thus, the blank non-lamella LC formulation itself did not show any penetration-enhancing effect. Permeation parameters were determined from the time course of the cumulative amount of the permeated drug. The calculated parameters are shown in Table 17.2. Partition coefficient, K, of calcein was markedly increased by application of the calcein entrapped in non-lamella LC-A formulation, suggesting that non-lamella LC-A could improve the calcein distribution into the skin by providing a high affinity to intercellular lipid structure in the skin (stratum corneum). Next, the time course of the skin permeation of calcein from the cubic liquid crystal dispersion (non-lamella LC-B) was evaluated; the results are shown in Fig. 17.6. Non-lamella LC-B as well as non-lamella LC-A enhanced the permeation of calcein through intact hairless rat skin; Table 17.3 summarizes the permeation parameters for non-lamella LC-B. Increased partition of calcein was observed by non-lamella LC-B, as by non-lamella LC-A. In contrast, no increase in drug partition was observed in stripped skin from non-lamella LC-B or from non-lamella LC-A. Non-lamella LC-A has a high affinity for the stratum corneum as well as the viable epidermis, whereas non-lamella LC-B shows high affinity for the stratum corneum, but not for the viable epidermis.