Fig. 36.1
Patient with wet necrosis extending to the deep fascia tissue and debridement was performed. Note the extent of necrosis after the debridement of all necrotic tissue
36.3.3 Dry Necrosis
Dry necrosis is a black, hard, mummified tissue often with clear demarcation from the surrounding tissue. It may have infection but usually is from severe ischemia resulting from vasculopathy. In cases where peripheral artery disease progresses slowly among ischemic-neuropathic diabetic foot patients, it gradually develops vascular compromise of the skin, and thus perception of ischemic pain is reduced [11]. The result is that the prevalence of claudication in the diabetic population with PAD is lower than the prevalence of critical limb ischemia (CLI) in this population. Thus eye inspection becomes a very important tool to diagnose the dry necrotic change among this group.
In diabetic patients with acute ischemia, sudden onset of pain is noted with pallor and coldness of the foot followed by mottling of the skin and shiny appearance of the texture.
Regardless of the duration of the peripheral artery disease, intervention of the artery is required to reperfuse the ischemic skin. Failure to reperfuse the foot will end in dry necrosis and loss of the tissue. Intervention angioplasty or bypass surgery should be applied accordingly to ensure better outcome after removal of dry necrotic skin [9]. Otherwise, further complication and increase in necrosis may occur in resection without vascular intervention. One must also be aware of the reperfusion injury after intervention. Once reperfused, inflammation will ensue and the wound around the dry necrosis will turn wet and increase the risk for infection. Debridement must follow reperfusion (Fig. 36.2).
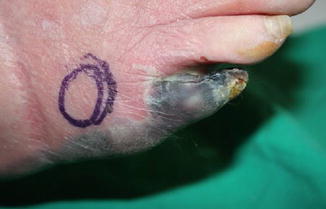

Fig. 36.2
Dry necrosis with relatively well-demarcated wound
36.4 Treatment and Reconstruction
When considering the diabetic foot for reconstruction, there are multiple issues to be addressed. These issues can be effectively approached though a multidisciplinary approach. The first step is to control the systemic aspect of diabetes. Then malnutrition, chronic renal disease, and hypertension have to be addressed properly and treatment schedules made before and after surgery especially hemodialysis and perioperative blood sugar control. While the systemic condition of the patient is being optimized, specific attention can be directed to the foot ulcer. Depending upon the general condition, peripheral vascular status; bone pathology; wound depth, location, and duration; involvement of chronic osteomyelitis; and patient motivation, wounds can be treated with debridement and other related surgical procedures. Another important issue concerns the vascular pathology of the patient. The vascular surgery consultation is warranted when the patient is symptomatic with ischemic pain or a nonhealing ulcer. Neuropathic ulcers require debridement of nonviable or infected tissue, combined with local wound care and off-loading. If the diabetic wound is not improved by such procedures or aggressive wound care, foot salvage procedures can be considered. A robust predictor of healing is 53 % change in wound area of diabetic foot ulcers [28]. In our center we monitor the change in wound size and depth, and when wound healing is stalled despite good standard of care such as off-loading, infection control, edema control, and advanced dressings, then additional treatment with hyperbaric oxygen, cell therapy, growth factor treatment, and negative pressure wound therapy is considered. But depending on the complexity of the wound, some of these secondary modalities are used primarily as well. Again, wound healing progress is closely monitored, and stalled healing despite of these multimodal therapies may become one of the indicators for reconstruction. Figure 36.3 shows the spectrum of care for diabetic foot ulcers from general care to reconstruction or amputation.
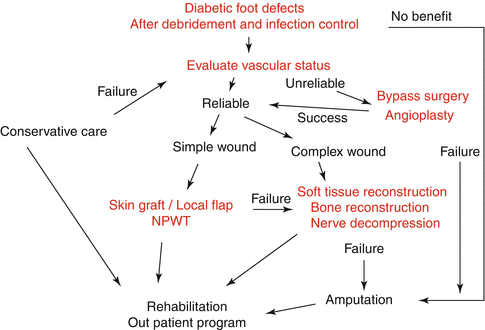

Fig. 36.3
Algorithm for diabetic foot reconstruction after debridement of necrotic diabetic wound
36.4.1 Debridement
The first step of treatment for diabetic foot wound is to evaluate, debride, and treat infection [3]. Missing timely management will lead to amputations and longer hospital days [26]. If symptoms and signs of infection are clinically suspected, proper treatment must be provided without delay. If superficial infection is suspected without systemic infection, antibiotic treatment along with non-weight bearing of the foot should be ensued. Optimal management of diabetic foot infection can potentially reduce incidence of major limb amputations and other related morbidities. All nonviable and infected soft tissue and bone should be excised during debridement. Milking along the proximal tendon can be helpful to identify and limit ascending infection. Tissue culture should be sent. Sufficient irrigation should follow after debridement to reduce bacterial count [4]. Recent advance in technology introduced a hydrosurgery system that allows to debride while preserving viable tissues and irrigate simultaneously, allowing reduced surgical time [15, 16]. Biodebridement such as maggot therapy has also been useful in clearing the necrotic tissue [10]. Application of negative pressure wound therapy has also played a role in reconstruction as they are used as an indicator and a wound preparation method of achieving clean wound with reasonable vascularity.
The understanding of vascular distribution of the foot, angiosome, helps to plan not only reconstruction but debridement [8]. When planning for reconstruction, one can avoid violating the angiosome territory when designing a local flap that may lead to flap breakdown [2]. Also by performing debridement according to the angiosome territory, one may enhance flap survival by increasing the chance for marginal vascularization from healthy surrounding angiosome territory.
Repetitive debridement should be performed as part of wound preparation for reconstruction while monitoring C-reactive protein for possible hidden infections and using it as an index for possible infection after reconstruction.
36.4.2 Vascular Intervention
In our surgical algorithm, all patients considered for microsurgical reconstruction undergo a noninvasive CT angiogram to evaluate the vascular status. The CT angiogram provides information regarding general vascular anatomy of the lower extremity and shows atherosclerotic change of vessels which is useful information when choosing recipient vessels. The overview is important as collateral vessels may be the main trunk to the distal limb. Without this information, one may elevate the flap harvesting the main arterial source to the distal limb and cause limb ischemia. If vascular status is in doubt, then revascularization by angioplasty or bypass surgery is referred. Although preoperative angiograms may indicate intact anatomy of the artery to the foot, actual findings upon surgery can be different. In order to confirm the distal vascular flow, we use ultrasound duplex scans. A study by Kim et al. showed correlation of peak blood flow velocity over 40 cm/s to flap survival [18]. However, in an ongoing study at our center, we hypothesized that the preoperative measurement of flow will not influence the survival of the flap, but the perioperative flow will play a more important factor. For now, ultrasound duplex scan provides information in the selection of recipient vessels or to refer for vascular intervention when no recipient vessels can be identified. The transcutaneous oxygen measurement (TcPO2) also plays a role in our protocol. Measurement over 30 mmHg in normobaric oxygen is a relative predictive factor for successful healing, whereas pressure less than that of 30 mmHg is likely to follow an unfavorable course [7, 14]. The wound, if measured under this level after vascular intervention, was treated with hyperbaric oxygen. If peri-wound TcPO2 measurements were over 30 mmHg, then further treatment including reconstructive procedures was planned; otherwise, amputations at according levels were performed. The ankle-brachial index (ABI) is not used as it is not reliable in diabetic patients due to the high incidence of calcified vessels causing falsely elevated values [13].
In diabetic patients, the most significant atherosclerosis occurs in the crural arteries often sparing the arteries of the foot [31]. Bypass to the dorsalis pedis or posterior tibial artery of the foot or angioplasty with or without stent placement procedures results in high success to restore perfusion pressure to the distal circulation of the foot reestablishing palpable pulse. The timing of when to perform reconstruction after vascular intervention is not clear. Reports have shown successful free flap transfer with simultaneous vascular reconstruction to salvage the limb [23]. But early bypass failures within 30 days are reported to be high [6, 29]. In our experience, partial flap loss or total loss was suddenly noted after 2–3 weeks in the cases combined with simultaneous or reconstruction following few days after vascular interventions. This may suggest that there should be a sufficient stabilization period after vascular intervention.
Reperfusion is most essential prior to microsurgery reconstruction. If vascular intervention fails and wound progresses, amputation is warranted.
36.4.3 Reconstruction Using Free Flaps
Once an adequate debridement and reasonable vascular perfusion are achieved, in extensive and complex diabetic foot defects, reconstruction should be considered. In my experience, local flaps such as reverse sural, medial plantar, or lateral supramalleolar for large defects have not been as successful as free flap reconstruction. Especially when reconstructing diabetic foot with reduced vascular flow, the utilization of local flaps may breach the distal flow of the small collateral vessels. One must consider current vascular status as well as future flow where small collateral vessels may play an important role for distal circulation. In this sense my choice for moderate and large defects is the reconstructive microsurgery to transfer free flaps. Inclusion criteria from a meta-analysis of free tissue transfer in 528 diabetic patients in 18 studies suggest (1) lower limb defect which has not displayed any signs of granulation or healing despite adequate debridement or necrotic tissue and conservative treatment, (2) no significant renal function impairment, (3) no significant systemic illness likely to be exacerbated by multiple operations and prolonged rehabilitation, (4) previously ambulatory with the aim to restore a functional limb, (5) likely to engage with the significant physiotherapy required for return to normal living, and (6) peak flow velocity of >40 cm/s in the recipient artery. We generally agree with the suggested inclusion criteria except for the significant renal disease. In our experience, we have not found an increased risk for failure despite the fact that uremia may cause a decrease in cell-mediated immunity and impair wound healing [5, 22, 32]. But we did report a significant risk of 4.857 times higher odds for flap failure in patients using immunosuppressive agents after renal transplant (p < 0.041) [22].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








