Fig. 6.1
Intercostobrachial nerve (preservation is not mandatory)
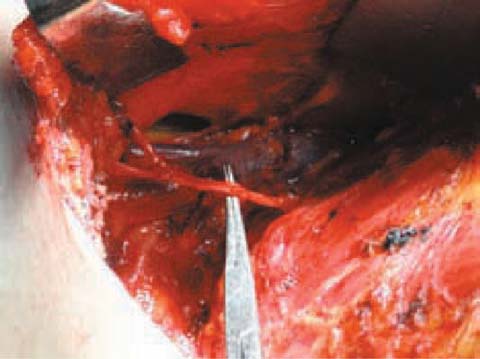
Fig. 6.2
Long thoracic nerve and thoracodorsal neurovascular bundle (their preservation is mandatory)
A drain (21 gauge or 10 mm Jackson-Pratt) is put in place, the incision is closed with a multi-layer suture and a compressive dressing is applied (Fig. 6.3).
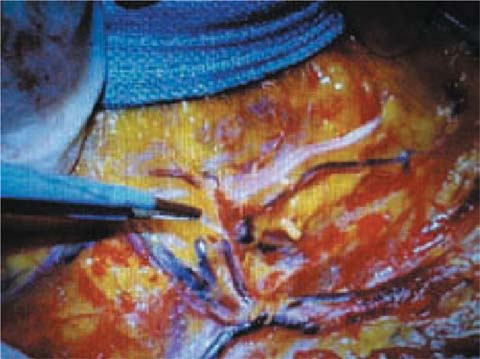

Fig. 6.3
Surgery field after ALND
6.2.2 Primary Axillary Lymph Node Dissection
The main goals of axillary surgery are:
1.
Local control
2.
Survival
3.
Staging.
6.2.2.1 Local Control
Axillary recurrence after primary ALND is very low (< 2%) [7–9]. The prognostical meaning of axillary recurrence is different if it is combined with distant metastasis (about 50% of the patients) [10].
If recurrent axillary node metastasis show up after primary ALND and it is the only recurrent site, prognosis is similar to that of a new diagnosed cancer with positive lymph node and salvage redo ALND (technically more difficult because of the scar tissue from the previous surgery) is usually curative [11].
6.2.2.2 Survival
In the past, most studies showed that patients who underwent ALND at the time of lymph node metastasis diagnosis had a lower overall survival (OS). This might had been because primary ALND was not performed at the time of breast cancer diagnosis and, most likely, because of the disease understaging, which involved avoiding adjuvant therapy.
On the other hand, recent studies show that ALND does not confer a survival benefit in the setting of early-stage clinically lymph node-negative breast cancer. In a 2009 meta-analysis, even though the axillary local recurrence rate is higher in patients that do not undergo ALND, the OS is not statistically different [12].
A 2011 meta-analysis, enrolling 8560 patients in eight randomized clinical trials, does not show statistically significant differences in disease free survival (DFS), OS and axillary recurrence for patients treated with ALND or (only) SLND, with axillary lymph node-positive or negative. Also SLND, compared to ALND, shows less postoperative complication and a better quality of life in the long term [13].
The neo and/or adjuvant therapy, hormone therapy and radiotherapy play a major role nowadays in the OS after axillary recurrence [14].
The primary ALND can improve DFS and OS in cN0 patients with lymph node metastasis that still have not a systemic hematic diffusion of the disease.
6.2.2.3 Staging
Some years ago a positive axillary lymph node result was considered the main risk factor for distant metastasis. The more lymph nodes that were involved, the higher was the risk. Systemic adjuvant therapy was strongly influenced by the number of axillary lymph nodes involved.
At the 2011 St. Gallen consensus conference, it was stated that the biological characteristics of the tumor play a major role in determining whether systemic therapies have to be used and that ALND is not needed anymore for staging [15].
Even though ALND has lost its former main staging role, the number of lymph nodes involved and the evidence of extra-capsular invasion of the nodes still influence the adjuvant therapy and radiotherapy.
Indications for primary ALND are:
Clinically positive axilla
Axillary node metastasis on fine needle aspiration (FNA) or core biopsy (CB)
Failed SLND
Positive SLN on intraoperative examination
Axillary local recurrence (ipsilateral or contralateral).
6.3 Sentinel Lymph Node
The sentinel lymph node/s is/are the first lymph node/s that drain the primary tumor. Anatomical studies showed that the lymphatic drainage of the breast starts from the deep part of the mammary gland (above the muscular fascia), moves to the cutaneous lymphatic system of the skin, especially around the nipple areola complex, and ends in the SLN.
6.3.1 Mapping
There are two validated techniques for SLN identification: blue dye (Patent blue dye, PBD) and/or a radioisotope (technetium, Tc99m). The latter is bound to a carrier, most commonly sulfur colloid in United States and colloidal albumin in Europe.
The identification success rate with blue dye alone varies from 65% to 90%, depending on the surgeon’s experience, and reaches 97% in combination with the radioisotope [16–18]. Using the radioisotope is definitely more demanding, both from the spending and organization point of view.
The cost of technetium is very high (with an exponential increasing trend); a nuclear medicine service and a nuclear doctor are required; surgery must follow radioisotope infiltration between 1 and 36 hours and a sensitive hand-held gamma probe must be available in the operating room [19].
On the other hand, the blue dye technique is cheaper (Fig. 6.4). The dye is injected in the subdermal plane, directly above the tumor, by the surgeon in the operating room, some time before the surgery. The volume of dye injected varies from 0.2 to 0.4 mL. All lymph nodes that show blue coloration are dissected (Fig. 6.5).
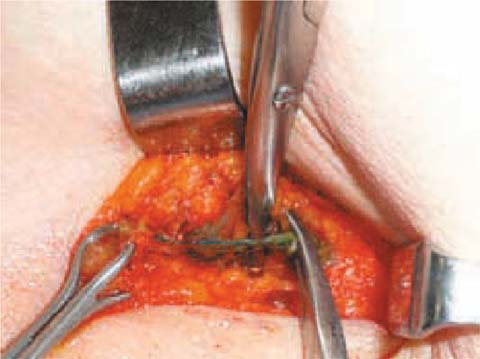
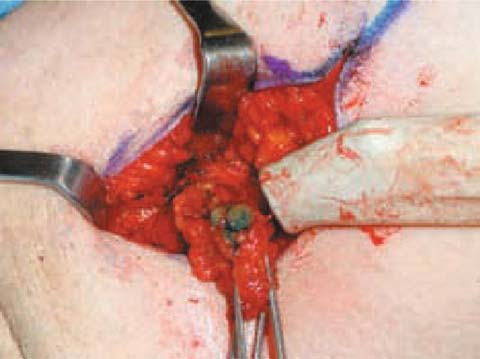

Fig. 6.4
Patent blue dye tracer allows lymphatic vessel identification

Fig. 6.5
The SLN is blue colored and hypercaptating (note handheld probe on the right)
Patients who undergo this technique show a transient bluish color of the skin and urine. A faint blue stain may persist at the breast injection site for as long as 1 year postoperatively. About 0.5% of patients have an anaphylactic reaction to the blue dye [20].
Fluorescent SLN mapping using green indocyanine (ICG) is currently being tested. When the vital fluorescent dye is injected around the areola, subcutaneous lymphatic channels draining from the breast to the axilla are visible by fluorescence; by tracking the fluorescence, it is possible to choose a better location for skin incision and find the SLN, which is the first lymph node that gets fluorescent (Fig. 6.6) [21].
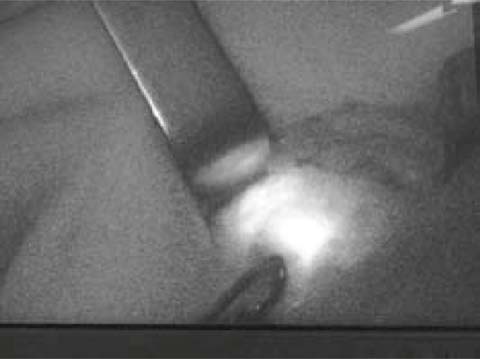

Fig. 6.6
Green indocyanine allows SLN identification by fluorescence (infrared probe visualization)
The cost of this technique is inferior to that using radionuclide and just a bit more expensive than using blue dye alone. A infrared probe is needed to visualize the fluorescence on the surgery site.
6.3.2 Site of Injection
The tracer (PBD, Tc99m or ICG) injection site can influence the SLN identification rate. Intratumoral injection has been abandoned because of the low identification rate related to the paucity of lymphatic vessels around the tumor, which causes a slow and sporadic migration to the SLN.
6.3.3 False Negative Scenario
The effect of the SLND false negative rate on the prognosis is unknown. An overview of 69 papers showed a 7% false negative rate for SLND followed by ALND [17]. However the axillary recurrence rate after negative SLND is less than 1% [25, 26], because other factors influence axillary recurrence (adjuvant therapy/radiotherapy of the axilla in the breast conserving technique, tumor biology and rapidly growing distant metastasis).
6.4 Sentinel Lymph Node Dissection
6.4.1 Technique
SLND can be performed under general anesthesia and under local anesthetic with intravenous sedation. Before starting surgery, blue dye is injected subdermally at a single site over the tumor. Using a hand-held gamma probe, the isotope injection site in the breast (radioisotope injected beforehand) is identified. The axilla is usually explored for SLN through a separate transverse skin line incision prior to the planned mastectomy or breast conservation procedure. As dissection is deepened through the axillary fascia, any blue lymphatics are left intact and traced proximally into the axilla, blue nodes are identified, and the gamma probe is used to identify any hypercaptating nodes. SLN are usually found low in level I, but in about 25% of cases they are found at other locations (along the latissimus dorsi muscle, near the axillary vein, beneath the pectoralis minor in levels II to III, as interpectoral or intramammary SLN).
The gamma probe is very useful throughout this dissection and is indispensable in patients with a very large or fatty axilla, when blue lymphatics or nodes are not found.
All blue SLN and hypercaptating SLN are removed; a median of 2–3 SLN per patient is submitted; when multiple hypercaptating SLN (or a diffusely hypercaptating axilla) are found, every effort must be made to remove the SLN with the highest count. All nodes with a count ≥ 10% of the highest count are submitted together with the SLN. The axillary incision after SLN biopsy is closed without drainage.
The morbidity from SLN biopsy is less than that of ALND but is not zero; patients may experience pain, seroma, hematoma, or infection.
6.4.2 When to Perform Sentinel Lymph Node Dissection
SLND must be performed in patients with diagnosis of invasive breast cancer obtained through: core biopsy (B5b), fine needle aspiration (C5), radiological finding (U5, R5) and definitive anatomopathological finding on the surgical specimen.
SLND can be avoided and ALND can be performed directly in U5 radiological patients with suspected metastasis [27]. If no metastasis are described SLND must be performed.
The SLND contraindications that still hold true are inflammatory carcinoma (T4) and a C5 diagnosis on any axillary lymph node’s FNA, the others (node diameter < 3 cm, multicentrical lesions, prior surgery and male breast carcinoma) have been removed.
Some contraindications, neoadjuvant therapy, pregnancy, “in situ” lesions and prophylactic mastectomy, are still under discussion.
In patients who undergo neoadjuvant therapy, the SLN identification rate is comparable to that of other patients, with a false negative value of 8% [28]; nevertheless the false negative value goes up to 25% if the SLND is performed in patients with proved metastasis at the diagnosis [29].
The biological meaning of a possible understaging related to a SLN negativization after neoadjuvant therapy is currently under discussion. The present indication is performing SLND before starting neoadjuvant therapy. However, SLND after neoadjuvant therapy is reasonable in cN0 patients.
The SLN identification rate during pregnancy and breast-feeding is just slightly inferior to the standard and the technique does not cause teratogenic effects. The onset of lactation must be pharmacologically blocked.
In the “in situ” carcinomas SLND must be performed only when the risk of a diagnosis of invasive carcinoma at the definitive pathology test is high (patients with a mass on clinical examination, G3 high-grade disease, distinctive radiological pattern and node diameter > 2.5 cm) and SLND should be performed in patients undergoing mastectomy (because mastectomy precludes it), in case invasive disease is subsequently discovered [30].
Performing SLND in patients undergoing prophylactic mastectomy is still controversial. The incidence of occult disease is low but patients with locally advanced or inflammatory primary breast cancer are at high risk for contralateral disease. This selected group of patients may benefit from SLND at the time of surgery but further studies are needed to prove it [31, 32].
6.5 Axillary Lymph Node Dissection after Positive Sentinel Lymph Node Dissection
When the SLN is negative, SLND alone with no further ALND is an appropriate, safe, and effective therapy in cN0 patients with breast cancer because OS, DFS and local control are statistically equivalent [33].
Although ALND is indicated when there is clinical evidence of disease in the axilla, it is still under discussion whether ALND should be performed in clinically silent or SLND diagnosed metastatic lymph nodes, and if this could positively influence the OS.
The classification of metastatic lymph node is based upon metastasis dimension:
1.
Isolated tumor cell clusters (ITC, small clusters of cells not greater than 0.2 mm, or single tumor cells, or a cluster of fewer than 200 cells in a single histological cross-section. ITC may be detected by routine histology or by immunohistochemical methods
2.
Micrometastasis (greater than 0.2 mm and/or more than 200 cells, but not greater than 2.0 mm)
3.
Macrometastasis (greater than 2.0 mm)
In the current TNM classification, ITC are defined as pN0(i+), they are not considered metastasis and therefore they should not be treated with ALND [34–36].
The clinical meaning of micrometastasis, classified as pN1mi, is currently unknown. Micrometastases are thought to have a smaller influence on OS and DFS among patients with early breast cancer.
In some studies, no statistically significant differences were observed in OS and DFS between patients diagnosed pN0 and pN1mi with SLND only [37–39




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






