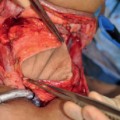
7 Although seemingly straightforward, the reconstruction of the scalp has required creativity and innovation from surgeons throughout medical history. Creating a balance of appropriate coverage of underlying structures and maintaining cosmesis is oftentimes challenging. The range of defects can be of the scalp alone to deficits of the scalp, bone, and dura. Calvarial and scalp defects require managing potential cerebrospinal fluid leaks in addition to contouring the skull asthetically.1 Matching skin thickness and color as well as maintaining hair-bearing skin are goals of therapy that are not always attainable. Beyond trauma and congenital deformities, oncologic resection is a specific aspect of reconstruction that forces surgeons to be resourceful. Delays in treatment can lead to tumor progression and greater reconstructive complexity. Therefore, delaying resection to allow for tissue expansion is not often recommended.2,3 Scalp reconstruction should provide durable coverage, preserve blood supply, and allow proper wound drainage without breakdown and calvarial exposure.3 The challenge of patients with previous surgical scars, pre- and postsurgical radiation, and dural invasion requires much thought before treatment to fulfill these ideals. The scalp can be a limiting factor in reconstruction. It is difficult to match in terms of thickness, color, and density of hair follicles. As a result, the best tissue type for scalp reconstruction is the scalp itself.1 Management historically focused on primary closure and the use of skin grafts to cover areas of granulation. Preservation of the pericranium allowed for better take of grafted skin. As early as the 1600s, calvarial perforation was suggested as a method of promoting granulation formation. The development of local flaps allowed for closure of defects and preserving hair formation and skin thickness. In the late 1960s and early 1970s, Orticochea4,5 described a four-flap and eventually a three-flap technique for closing large scalp defects. These flaps enabled greater success at wound closure and preservation of hair-bearing tissue. For nononcologic reconstruction, the use of tissue expansion has allowed closure of large defects and maintaining some hair-bearing potential. Regional flaps were found to be useful for reconstruction of occipital defects, but are limited in cosmesis. The challenges of reconstruction in the oncologic patient as well as a method of covering large surface areas of the skull were met with the development of microvascular reconstruction. Free tissue transfer has enabled surgeons to correct for previous surgeries, wound breakdown, and radiation therapy and still manage to cover entire scalp defects. The scalp is composed of five distinct layers: skin, subcutaneous tissue, galea aponeurosis, loose areolar tissue, and the periosteum of the skull. The skin is the thickest compared with that on other areas of the body. The subcutaneous tissue contains most of the blood supply and lymphatics. The galeal layer is the strength layer and provides the most limitation of scalp movement. It attaches to the fascia overlying the frontalis muscle anteriorly, the temporoparietal fascia laterally, and the occipitalis muscle fascia posteriorly. Deep sutures at this level help alleviate tension along the skin.6 Epicranial muscles lie between the loose areolar tissue and the galea.7 Mobility is greatest at the parietal regions of the scalp where the temporoparietal fascia overlies the temporalis fascia.1 Advancement from this area allows the most gain for rotational flaps. To help increase length, galeotomies can be used. Galeotomies at 1-cm intervals can decrease tension by 40% and gain 1.67 mm per incision.8 The cuts should be parallel to the blood supply to avoid devascularization, as the vessels are immediately superficial to the galea.6,9,10 Compromise of blood supply can lead to alopecia as well as necrosis of reconstructive flaps.10 The blood supply of the scalp is composed of branches of the internal and external carotid arteries. The anterior scalp receives its vascularization from the supraorbital and supratrochlear arteries. The superficial temporal artery and the posterior auricular artery supplies the scalp laterally and posterolaterally, respectively. The superficial temporal artery supplies the greatest region in the scalp and branches into frontal and parietal vessels.7 The posterior aspects of the scalp receive its blood supply from the occipital artery above the nuchal line. Below this area is supplied by the perforators of the trapezius and splenius capitis muscles. Collateralization between these distinct territories is extensive. The use of local flaps should incorporate one of these major vessel systems to ensure adequate vascularization. Venous drainage from the frontalis, parietal, and occipital veins matches their corresponding arteries and drains the frontal, lateral, and posterior scalp into the external jugular vein.7 Neural innervation of the scalp is derived from a combination of cranial and cervical nerves. The anterior aspect is supplied by the first branch of the trigeminal nerve through the supraorbital and supratrochlear nerves. Laterally, the zygomaticotemporal, auriculotemporal, and the lesser occipital nerves provide innervation from the second and third branches of the fifth cranial nerve. Along the posterior scalp, cervical branches from C2 and C3 provide branches for the lesser and greater occipital nerves. Superiorly, the scalp is supplied by the third occipital nerve and is a branch of C3. In general, the pericranium of the vertex of the skull is less sensitive than the inferior areas. Neither the bones nor the veins of the skull receive any proprioception or nociception.7 The size, depth, and location of the defect have the greatest impact as to what type of reconstruction should be used for scalp reconstruction. Although no one staging system exists, various algorithms have been suggested throughout the literature. Beasley et al11 describe a staging system based on size and suggest potential reconstruction options. Scalp defects less than 200 cm2 are classified as stage IA. Stage IB defects are the same size as stage IA, but they are associated with associated risk factors for failure, such as heavy trauma, infection, previous radiation, or a history of failed closure. Stage II defects are 200 to 600 cm2 in size. Stage III defects are larger than 600 cm2. Primary closure or local flaps are recommended only for stage IA defects, whereas free flaps are recommended for all other stages.11 Leedy et al1 differentiate defects by size and location. Anterior defects that are small and do not affect the hairline can be closed primarily. Rotational flaps are recommended to preserve or restore the hairline. Moderate (up to 25 cm2) and large defects (>25 cm2) can be closed with rotational advancement flaps and temporoparietal flaps, respectively. Tissue expansion and Orticochea flaps are also useful. For larger parietal defects, tissue expansion is required. Skin grafting over muscle and rotational flaps are other viable options. For large occipital lesions, Orticochea flaps and tissue expansion are useful as well as rotational flaps for smaller lesions that cannot be closed primarily. For vertex lesions, Leedy et al recommend primary closure for small lesions. For lesions less than 4 cm in width, closure with galeal scoring or a pinwheel flap is suggested. Otherwise, large rotational or advancement flaps with back grafting or tissue expansion are needed. Leedy et al recommend free tissue transfer for patients with neartotal scalp defects. Iblher et al2 reviewed defects from oncologic resections and created an algorithm for closure based on size and location as well. For defects less than 3 to 4 cm in size, primary closure is recommended. If the defect is less than 6 to 8 cm (4 to 5 cm near the hairline), a split-thickness skin graft or local flap is advocated. Defects less than 8 to 10 cm necessitate free flap closure. If the larger defect is occipital, regional flaps are potential candidates for reconstruction. Although opinions vary on when to use which reconstructive option, a thorough understanding of each is necessary before tailoring a treatment plan for an individual patient. If primary closure cannot be achieved, even with the aid of galeotomies, several options are available to aid in reconstruction. Healing by secondary intention and allowing granulation of a defect is one method of closure. This technique requires meticulous care of the defect over several weeks of healing. However, it requires no additional surgery, does not require a vascular pedicle, and allows easy detection of tumor recurrence.12 This technique is useful in patients with multiple medical comorbidities that may impede wound healing. Split- or full-thickness skin grafting can be used over the granulation bed. Skin grafting over the defect is another method of closure. It is helpful to graft over areas of granulation, intact pericranium, or muscle to allow for the greatest chance for the graft to take. Burring down the outer layers of the calvaria and revealing the diploic space can be useful to help with vascularization if the pericranium is not viable.3 The ideal area to graft are nonirradiated defects with a good vascular bed and no need for postoperative radiation.3 Split-thickness skin grafts can cover a large wound area and allow for easy detection of tumor recurrence.11,12 Thinner grafts have a better chance of survival due to less nutritional requirements; however, they are not as durable as other modalities of closure. To improve graft survival on previously radiated bone that granulates slowly, galeal flaps can be used. These flaps rotate or transpose galea from sites adjacent to the defect and improve skin graft take by providing a vascular bed.13 Split grafts often are not cosmetically advantageous, with poor color and poor thickness matching. If meshing of the graft can be avoided, the appearance can be improved, but will sacrifice coverage surface area. Tissue expanders follow the tenet of replacing scalp with scalp and are useful in patients not limited by time constraints for reconstruction. As a result, the oncologic patient is not the best candidate for the weeks necessary to allow adequate expansion time.2,3 The process involves implanting an expander and inflating the device over several weeks. A fibrous capsule forms around the expander, allowing an increase in blood supply to the scalp.6
Scalp Reconstruction
♦ RELEVANT ANATOMY
♦ CLASSIFICATION OF DEFECTS
♦ OPTIONS FOR RECONSTRUCTION
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree