(1)
RZANY & HUND Privatpraxis für Dermatologie und Ästhetische Medizin, Kurfüstendamm, Berlin, Germany
8.1 Introduction
8.2 Epidemiology
8.4.4 The Biofilm Theory
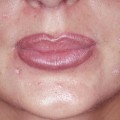
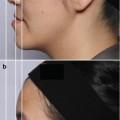
8.5.1 Bluish Discoloration
8.5.3 Acute Vascular Reaction
8.5.4 Nodule Formation
8.5.5 Abscess Formation
Abstract
Benign asbestos-related pleural diseases are the most common pathologic and clinical abnormalities related to asbestos exposure, with a greater prevalence than asbestosis. Solomon et al. [1] emphasized that the pleural manifestations of asbestos exposure include four specific benign pleural reactions: (1) benign asbestos effusion, (2) parietal pleural plaques, (3) diffuse pleural fibrosis, and (4) rounded atelectasis, or an area of collapsed, airless lung adjacent to an area of visceral pleural fibrosis. Notably, there is considerable overlap among these four disease processes (Fig. 6.1), with various combinations manifesting simultaneously or sequentially in a single individual. For example, a patient with benign asbestos effusion may subsequently be found to have diffuse pleural fibrosis, or a patient with parietal pleural plaques may develop rounded atelectasis.
8.1 Introduction
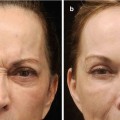
A multitude of adverse reactions can occur after the injection of fillers. It is important to understand that there is NO risk-free filler. Even when using biodegradable fillers with a good scientific background, adverse reactions may occur. Fortunately, severe adverse reactions as nodule formation or even ulcerations are rare for most fillers.
In general, adverse reactions can be grouped depending on time after onset into acute, subacute, and delayed reactions (Table 8.1). “Subacute” is somewhat vaguely defined; it basically means something that happens in the weeks after injection.
Table 8.1
Possible adverse reactions to fillers
Immediate reactions (within 72 h after injection) (more common) | Transient erythema |
Transient edema | |
Transient induration | |
Transient pruritus | |
Transient ecchymosis | |
Subacute reactions (rare) | Abscess formation |
Discoloration (i.e., bluish) | |
Persistent local symptoms (hypersensitivity reactions): erythema, edema, induration, pruritus, hyperpigmentation | |
Local necrosis | |
Reactivation of herpes | |
Delayed reactions (rare) | Nodule formation |
Abscess formation | |
Ulcerations |
Key Points
Every injectable filler can elicit an adverse reaction.
Adverse reactions can be grouped according to their occurrence after the initial injection.
Basically we can distinguish between acute, subacute, and delayed reactions.
8.2 Epidemiology
Adverse reactions may be also grouped into frequent and rare ones. The frequent reactions are easy to detect, especially if they are acute, e.g., if you see an immediate swelling during lip augmentation. If the event is rare and delayed, it is much more difficult to detect.
Having good safety data from a clinical trial does not necessarily mean that you have a safe filler in your hands. Clinical trials usually cover a time period ranging 6–12 months. Anything beyond that would require separate reporting (Strom 1994).
So how do we get information about these reactions? There are three basic sources: (1) the companies, (2) the agencies (e.g., the BfArM, the FDA), and (3) specialized adverse reaction registries as the Berlin registry (Zielke et al. 2008). However, one must be aware that these sources only collect the data. We, the treating physicians, must actually report the data.
Do’s
Communicate and/or report adverse reactions and their therapy.
Key Points
Frequent and acute adverse reactions are easy to detect.
Rare and delayed reactions require much more effort.
Only if we report the adverse reactions we do see these reactions will become known.
8.3 Identification of the Responsible Filler
In an ideal world, the patients know with which filler they were injected. In reality, this is often not the case. Patients rarely remember the name or might confuse the filler with a popular brand name, e.g., sometimes Restylane is used synonymously with filler.
How can this issue be solved? A thorough past history analysis, which might include going back to the treating physician, might be the first step. If this is not helpful, the next step is a biopsy. The biopsy may allow identification of the group of fillers, e.g., biodegradable versus permanent as well as specific fillers as PLLA. However, not every pathologist is familiar with this task. A diagnosis of “foreign body reaction” is only partially helpful. If the pathologist seems to be insecure, the paper from Dadzie et al. (2008) might be helpful.
Key Points
Identification of the causal filler is very important.
In case of uncertainty (e.g., nodules are suspicious of an adverse reaction to Dermalive but only biodegradable filler injections have been reported), a biopsy is recommended.
Be aware the biopsy might distinguish between groups of fillers but not between fillers of one group (e.g., the different HA fillers).
8.4 Potential Risk Factors
What are the risk factors for these reactions? We would be pleased to know them because no decent physician would like to have one of his patients develop one of these reactions. However, most of the risk factors have remained unknown thus far. The main reason is that they are difficult to study, as most of the reactions are rare and delayed. Therefore, investigating them would require the efforts of multicenter case-control studies with a duration of at least a couple of years.
In summary, present knowledge is based on case reports and case series and therefore needs to be viewed cautiously.
Roughly three groups of risk factors can be distinguished:
1.
The doctor
2.
The product
3.
The patient
8.4.1 The Doctor as a Risk Factor
When do we as doctors become a risk factor? (1) When we use products of questionable origin, (2) when we inject the wrong product for the wrong area or when we inject too aggressively (there is some evidence that arterial embolization might be caused by high-pressure injections), (3) when we insufficiently dilute the product (see PLLA), and (4) when we do not do the local disinfection properly prior to the injection – especially when we are using cannulas. Furthermore, this has been reported before – when we share syringes between different patients. This might sound economical, but might be associated with an increased risk of infections.
8.4.2 The Product as a Risk Factor
Substances with a rough irregular surface seem to have a higher risk for adverse reactions. There are basically three examples for that: (1) PLLA, which has a strong tissue-stimulating ability especially when not correctly diluted upon injection (Rossner et al. 2009b); (2) hydroxyethylmethacrylate in a fixed combination with hyaluronic acid (Dermalive) (Rossner et al. 2009a), which to our best knowledge is currently for the sake of everybody no longer being produced; and (3) polymethylmethacrylate and collagen, when modifying the surface structure of the rate of adverse reactions could be reduced. There are also some suggestions that some HAs might have an increased risk of adverse reactions. Be specifically suspicious of HAs where no good clinical trial data exists (e.g., this is true for the majority CE-marked HA products in Europe).
8.4.3 The Patient as a Risk Factor
When is the patient a risk factor? As said before, we do not know a lot about the origin of these adverse reactions. However, there is some evidence that active autoimmune diseases, such as rheumatoid arthritis, or a treatment with interferon might increase the risk of adverse reactions specifically when using a tissue stimulation filler such as PLLA.
Do’s
Take a thorough past history in your aesthetic patients.
If a patient reappears in your office for a reinjection, take a few minutes and ask him/her if a new medical condition did arise.
Don’ts
Do not do routine laboratory screening tests in your patients. There is no evidence that they are helpful.
Do not do skin testing (except for bovine collagen) as skin testing would only make sense in patients with a delayed-type allergy.
Key Points
There is no sense of an undirected laboratory screening for autoimmune diseases prior to an injection.
However, it is highly advisable to ask prior to the injection the patient if he/she is aware of any current medical conditions.
8.4.4 The Biofilm Theory
The biofilm theory is momentarily quite en vogue. The theory comes from a group of Danish physicians working on adverse reactions to polyacrylamide and who discovered bacterial proteins next to polyacrylamide depots. It basically states that bacteria, either from the injection or from an infection close to the area of the injection, and the filler form matrix-enclosed aggregates, termed biofilm. The biofilm will then trigger a foreign body reaction that is difficult to treat. The administration of steroids is considered contraindicated as it might lead to abscesses (Bjarnsholt et al. 2009). The Bjarnsholt et al. (2009




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree