Environment or purpose of use
Product
Medical
Protection gloves, finger cots, catheters, tubes, stopper, sealings, splints, wound dressings, bandages, condoms, hot water bag, implants (mostly silicone)
Laboratory
Protections gloves, Peleus (pipet) ball, stopper
Construction
Cable material, rubber grips of tools, sealing, insulation, hoses, buckets
(Vehicle) production and repair
Tires, rubber grips of tools, cables, insulation
Cleaning
Gloves, rubber sponge, hoses
Household
Rubber bands, cell phone covers, kitchen devices, baking and ice cube molds (mostly silicone)
Sport
Balls, mats, flooring, handles of sport instruments, diving equipment and wet suits, swimming goggles, currycomb
Clothing
Bras, waistband of trousers, cuffs, socks, stockings, suspenders, wristbands
Shoes
Sport shoes, rubber boots, shoe soles
Toys and children’s items
Dolls, ducklings, balls, erasers, swings, pacifiers, craft supplies (e.g., for making of wristbands; e.g., Loom (mostly silicone))
Table 14.2
Frequent contact allergens (rubber additives added to natural or synthetic rubber during the manufacturing process)
Rubber additive | Contained in rubber | Other exposures | Impaired occupational fields |
|---|---|---|---|
Thiurams | Yes (e.g., protection gloves, rubber form products (e.g., tires, hoses, sealing rings, clothing)) | Pesticides, fungicides, germicides, insecticides, insect repellents, preservatives (wood, paints, greases, etc.) | Rubber production, productive industries with unavoidable contact to rubber form products (e.g., assembly lines, tires, hoses) |
Tetraethylthiuram disulfide (TETD, disulfiram) as medication (Antabus®) for alcohol withdrawal and as chelating agent used for nickel intoxication | Production of pesticides, farming; floristry may be impaired, if thiuram-containing fungicides cannot be avoided | ||
In the medical field, in construction; for cleaning and hairdressing, most frequently thiuram-free protection gloves may be used as a surrogate | |||
Mercaptobenzothiazole and its derivatives | Yes (e.g., protection gloves, shoe soles, tires, industrial rubber) | Glues (neoprene based), antifreeze, automotive cooling systems, refrigerants, cutting fluids/greases, detergents (granulated and tablets), paint, fungicides, pesticides, germicides, veterinarian medicaments, leather industries and shoemaking | Leather processing industries, shoe and rubber production. Metal industries may be impaired if MBT-containing cutting fluids cannot be exchanged |
In the medical field and construction, most frequently MBT-free protection gloves may be used as a surrogate | |||
Dithiocarbamates | Yes (e.g., protection gloves, medical products, condoms, rubber boots, rubber covered tools, sealings, cable insulation) | Fungicides (zinc dimethyldithiocarbamate (Ziram), zinc ethylene-bis-dithiocarbamate (Zineb), Maneb (mangan-ethylene-bis-dithiocarbamate)) | Rubber production, productive industries with unavoidable contact to dithiocarbamate-containing rubber form products (e.g., assembly lines, tires, hoses) |
Farming and gardening, as well as production and processing of biocides may be impaired | |||
Thioureas | Yes (e.g., neoprene products (e.g., wet suits, other sport equipment), thermoplastic coatings, foam rubber products) | Anticorrosives, antioxidants, acidic detergent, cleaning products, paint/glue remover, fungicides, pesticides, PVC adhesives/tapes | Rubber production, productive industries with unavoidable contact to thiourea containing products |
N-isopropyl-N′-phenyl-phenylenediamine (IPPD) | Yes (used as antioxidant and antiozonant agent in statically and dynamically highly challenged natural or synthetic rubber products; mostly in the industrial environment; gives the black color to industrial rubber; e.g., in tires, car parts, conduction belts, cable insulation, hoses, and tubes, sealings; milking machines; protection and diving gear). Non-occupational exposures are rare: squash balls, motorbike handles, wrist watch bands, eyelash formers, orthopedic supports, underwear | Rubber cement, acrylates, gasoline, cross-reactive components in hair dyes | Black rubber production and assembly lines (tools with covered handles, tubes, hoses, tires.), car repair (with contact to black rubber tubes and tires) |
14.1.2 Types of Rubber
Rubber elastomers can be divided in the following classes [22]:
(i)
General-purpose rubber: natural (NRL), polyisoprene, styrene-butadiene, butyl, ethylene-propylene, and polybutadiene rubber
(ii)
Solvent-resistant rubber: polysulfides, nitrile, polychloroprene, polyurethanes, and epichlorohydrin rubber
(iii)
Heat-resistant rubber: silicone, chlorosulfonated polyethylene, polyacrylates, and fluoroelastomers
Nowadays, natural rubber latex supplies 25 % of the rubber market, whereas synthetic rubbers constitute the remaining 75 % [5]. Blends between natural and synthetic rubber materials exist [5]. Styrene-butadiene is now the major synthetic rubber produced. In comparison with natural rubber, it is weaker and less resistant to fatigue, but it has the merit of ageing more slowly [22]. Since most rubberized materials are unlabeled, it is difficult to determine whether a product contains natural or synthetic rubber [5]. The existing overlap between ingredients in “rubber” and “plastic” further complicates the matter [5]. Whereas completely cured plastic materials are rare sensitizers, fully cured rubber products produce allergic reactions since the sensitizers in rubber can leach out over time [5].
14.1.3 Rubber Components
Two main groups of compounds different in nature have to be distinguished as allergen sources in rubber: (1) proteins from natural rubber latex (NRL) which may lead to type I allergies (presenting as contact urticaria and rarely also protein contact dermatitis) and (2) rubber additives which are added to natural rubber latex as well as to synthetic rubber elastomers during the manufacturing process (e.g., vulcanizing agents (e.g., sulfur or sulfur donors, organic peroxides, phenol resins, metal oxides), accelerators (e.g., thiurams, benzothiazoles, guanidines, dithiocarbamates), activators (e.g., zinc oxide), retarders (e.g., organic acids, cyclohexylthiophtalimide, N-nitrosodiphenylamine), fillers (e.g., China clay), antidegradants (antioxidants (e.g., phenylenediamines, quinolines, hydroquinones, butylhydroxytoluene (BHT), phosphites), antiozonants (e.g., PPD derivatives)) to enhance the technical properties of the final product, plasticizers (e.g., phthalate esters in rubber tires), processing aids (e.g., mineral oils, solvents, talc), tackifiers, stabilizers (e.g. casein), pigments (inorganic pigments and organic dyes and lacquers), among others) [22, 5], some of which may lead to type IV allergies (allergic contact dermatitis). Hundreds of different rubber additives may be used in different blends; in a particular rubber product, however, around a dozen different components may be used [22].
Vulcanizing agents are necessary to induce cross-linking of natural as well as synthetic rubber elastomers during the process of rubber manufacturing [9, 22]. The most common vulcanizing agent in general-purpose use is sulfur. Common sulfur donors are morpholine, dithiocarbamates, dithiophosphonates, and tetraethylthiuram disulfide and tetramethylthiuram disulfide [30]. The reaction between sulfur donors and rubber is slow. To speed up the process, a group of chemicals is used as accelerators: slow accelerators are thiourea derivatives and amines; moderately fast accelerators are 1,3-diphenylguanidine, mercaptobenzothiazoles, and sulfonamides; very fast accelerators are thiurams, dithiocarbamates, and thiophosphates [30]. While some synthetic rubbers (e.g., butyl and nitrile) can be polymerized with organic peroxides without the addition of sulfur, others (e.g., styrene-butadiene) require much greater amounts of sulfur donors (e.g., 2-MBT, thiurams) than natural rubber [5].
However, silicone rubber, which is fully saturated, cannot be vulcanized with sulfur or sulfur donors. Instead, peroxides are necessary to achieve cross-linking [30]. Silicones are relatively nonreactive and highly biocompatible. Hypersensitivity reactions to silicone polymers have only rarely been reported [37].
14.1.4 Most Important Rubber Allergens
In patients with suspected rubber allergy, contact allergies (type IV allergies) to rubber additives are frequent, whereas type I allergies (presenting as contact urticaria syndrome) to natural rubber latex (NRL) proteins are much less frequent.
14.1.4.1 Type IV Allergens: Rubber Additives
The rubber accelerators (thiurams, carbamates, thiazoles and thioureas) and antioxidants (mainly derivatives of PPD) constitute the most frequent contact allergens among the rubber chemicals; reactions to other components of rubber (except for phenol formaldehyde resins (used as tackifiers/reinforcing agents) and epoxy resins (used as stabilizers) are rare [5]. The accelerators cause the greatest amounts of sensitivity among users of rubber products (Fig. 14.1); in contrast, workers involved in the manufacture of rubber are more likely allergic to the amine antioxidants (e.g., IPPD) [5]. Allergic reactions to the synthetic rubber monomers/polymers themselves may occur and, however, are very rare (Fig. 14.2).
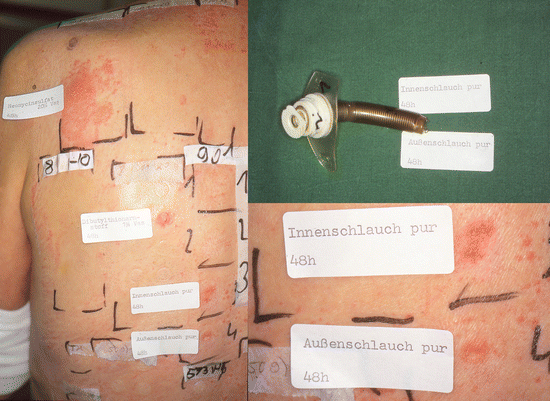
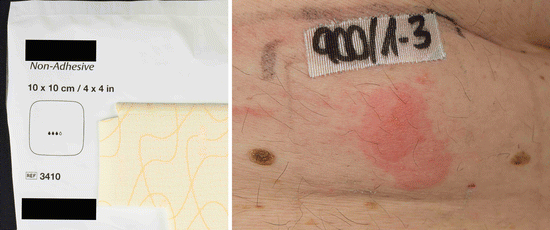

Fig. 14.1
Positive patch test reactions to dibutylthiourea, inner tube (Innenschlauch), and outer tube (Außenschlauch) of the tracheal cannula causing allergic contact dermatitis in a 56-year-old female patient with tracheostoma following surgery for hypopharyngeal carcinoma 6 year earlier. Additionally, a type IV sensitization to neomycin was diagnosed

Fig. 14.2
Positive patch test reactions to a polyurethane wound dressing causing acute allergic contact dermatitis in a 70-year-old male patient. According to the manufacturer, no accelerators are used during the production process, and this case was the first case of contact dermatitis to this kind of wound dressing ever reported. The patient exhibited concomitant type IV sensitizations to several rubber chemicals (mercapto mix (CBS, MBTS, MOR) without MBT, 1,3-DPG, cyclohexylthiophtalimide, tert-butyl hydroquinone) which were after meticulous research of the manufacturer not used during the production process. A rare case of type IV sensitization to the polyurethane polymers may be assumed
Thiurams and Dithiocarbamates
Thiurams are still the most frequently recognized rubber accelerator [15, 17, 31] with prevalences of sensitization to the thiuram mix between 2.0 and 2.7 % in patch test clinics throughout Europe, with exception for Italy, Lithuania, and the Netherlands where it is considerably lower. The thiurams used industrially include tetramethylthiuram monosulfide (TMTM), tetramethylthiuram disulfide (TMTD), tetraethylthiuram disulfide (TETD), and dipentamethylenethiuram disulfide (PDT).
In a recent analysis of data from the ESCCA network, contact allergens with the strongest association to occupational dermatitis (i.e., those with a risk of occupational dermatitis ≥1.75) were thiurams, epoxy resin, mercapto rubber chemicals, and N-isopropyl-N′-phenyl-p-phenylenediamine (IPPD), followed by a number of antimicrobials. Concordantly, thiurams, mercapto rubber chemicals, and IPPD were defined as predominantly occupational allergens [27].
As occupational subgroups mainly at risk of contact sensitization to thiurams except for rubber industry workers, healthcare workers (physicians, nurses, and related), food processors (cooks, meat and fish processors), and professional cleaners were identified [32]. Whereas between 1992 and 2006 a significant decline of sensitization prevalence could be identified in healthcare workers, no significant trend was determined in food processors and professional cleaners [32]. A predominance of exposure via gloves was illustrated by the pattern of sites associated with an increased risk; however, footwear also seems to have some relevance for elicitation of contact dermatitis due to thiurams [32].
Thiurams, dithiocarbamates, and mercaptobenzothiazoles have fungicide effects and for this reason are used in agriculture. They have been also described in adhesives, paints, cutting oils, and veterinary medications [5]; however, these exposures seem to be outdated in the European Union [12]. Due to its potential carcinogenicity and known sensitizing potency, 2-mercaptobenzothiozole is not being used anymore in cutting oils in Germany [http://www.kss-komponenten.de/, last accessed 20 Dec. 2014].
Currently, none of the veterinary medications listed in the EudraPharm weblist (European Union Drug Regulating Authorities Pharmaceutical Database; summarizes all medicinal products authorized in the European Union; http://www.eudrapharm.eu/eudrapharm/) contains thiurams, dithiocarbamates, or mercaptobenzothiazole. The exposure may vary in countries outside the EU. In the Green Book (FDA-Approved Animal Drug Products, Sect. 2.0 – Active Ingredients), one 2-mercaptobenzothialzole-containing product for the treatment of dogs is listed (Sulfodene ™ medication for dogs), whereas no thiuram- or dithiocarbamate-containing veterinary drugs were found (http://www.fda.gov/AnimalVeterinary/Products/ApprovedAnimalDrugProducts/UCM2006464; last accessed 20 Dec. 2014).
Tetraethylthiuram disulfide (i.e., disulfiram; Antabus ™) has also been used as an oral medication to support the treatment of chronic alcoholism by producing an acute sensitivity to alcohol. According to EudraPharm weblist (last accessed 20 Dec. 2014) in Europe, Antabus™ is currently only still available in Finland.
Positive patch test reactions to thiurams are frequently combined with positive patch test reactions to dithiocarbamates [6, 15]. Even though the use of thiurams as vulcanization accelerators in rubber glove production has been reduced and dithiocarbamates and mercaptobenzothiazole derivatives are now more commonly used [15, 21], positive patch test reactions to thiurams still are more common than positive reactions to dithiocarbamates [17, 31]. A possible explanation to this is that thiurams and dithiocarbamates constitute a redox pair in which a dithiocarbamate may oxidate into corresponding thiuram disulfide, and the thiuram may be reduced to reform the dithiocarbamate [6, 21]. Thiurams are considered to be better markers for sensitization to the dithiocarbamate/thiuram redox pair than the dithiocarbamates [21].
Historically, the predominant use of carbamates has been in pesticides and fungicides; however, during the last decade, the use as rubber chemical, especially in nitrile gloves, has increased [5]. Sodium dithiocarbamates are water soluble, whereas zinc dithiocarbamates are water insoluble. From the latter group zinc diethyldithiocarbamate (ZDEC), zinc dibutyldithiocarbamate (ZDBC), zinc dimethyldithiocarbamate (ZDMC), and zinc dipentamethylendithiocarbamate (ZPC) are clinically relevant contact allergens frequently contained in elastomers [30].
The prevalences of sensitization to ZDEC (derived from patch test clinics of the ESSCA network where it was tested as supplement to the standard series) varied from 0.3 % in Finland to 1.0 % in Switzerland [31].
Thiazoles
Thiazoles are derivatives of benzothiazoles compounded with sulfenamides [5]. The benzothiazoles include 2-mercaptobenzothiazole (MBT), dibenzothiazyl disulfide (MBTS), and the zinc salt of 2-mercaptobenzothiazole (ZMBT); the sulfenamides include N-cyclohexyl-2-benzothiazyl sulfenamide (CBS), N-tert-butyl-2-benzothiazyl sulfenamide (TBBS), and 2-(4-Morpholinyl mercapto) benzothiazole (MOR, MBS; MMBT). MBT, MBTS and CBS are the more widely used thiazoles [5]. Their use has increased in gloves during the last decade and MBT remains the most widely used accelerator for industrial rubber [5]. MBT was found to be the most frequent sensitizer in patients with shoe dermatitis [1].
The prevalences of sensitization to thiazoles are less frequent than it is to thiurams and dithiocarbamates. The prevalences of sensitization to MBT derived from patch test clinics of the ESSCA network varied in the different countries from 0.2 % in Lithuania to 1.3 % in Austria and Poland; the prevalences of sensitization to the mercapto mix (without MBT) varied from 0 % (Finland) to 1.0 % in Austria [31].
Thioureas
Thioureas include dibutylthiourea (DBTU), diethylthiourea (DETU), diphenylthiourea (DPTU), and ethylene thiourea (ETU). They are used in the production of synthetic rubbers, particularly neoprene products and foam rubbers [3, 23, 5]. Thioureas are only rarely used as accelerators in protective rubber gloves [17]. The most frequent source of relevant positive patch test reactions have been reported to be shoes and medical devices (Fig. 14.1) before gloves [9]. Allergic contact dermatitis to thioureas has occasionally been noted from exposure to rubber, especially neoprene. Thiourea accelerators may decompose to give isothiocyanates [22].
p-Phenylenediamine Derivatives
Among over 100 existing antioxidants, the most important sensitizers are phenylenediamine derivatives: N-isopropyl-N′-phenyl-4-phenylenediamine (IPPD), N-phenyl-N′ cylohexl-4-phenylenediamine (CPPD), N-N′ diphenyl-4-phenylenediamine (DPPD), and N-(1-3 dimethylbutyl)-N′-phenyl-4-phenylenediamine (DMPPD). They are contained in industrial rubber and rubber of black color. Although they are strong sensitizers, the sensitization prevalence to phenylenediamine derivatives is low probably due to automation in the production process [5]. IPPD is included in the baseline series and is an uncommon contact allergen with sensitization prevalences ranging from below 1 % to 1 % [31].
14.1.4.2 Type I Allergens: Natural Rubber Latex Allergens
Of the more than 240 natural rubber latex (NRL) polypeptides, 15 latex proteins (Hev b 1–15) have been officially recognized as allergens by the International Union of Immunological Societies (IUIS) (Table 14.3). Their clinical relevance and connection to the latex-fruit syndrome (cross-reactivity with homologous proteins contained in exotic fruits) have been reviewed [in 36]. Recently, Hev b 1, 2, 5, 6.01, and 13 were identified as major allergens in differently exposed subgroups [28]: Hev b 2, 5, 6.01, and 13 were identified as the major allergens (1) in latex-allergic healthcare workers (HCW) and (2) combined with Hev b 1 and Hev b 3 in latex-allergic patients with spina bifida (SB). (3) In latex-allergic patients without spina bifida who had undergone multiple surgeries (MS), only nHev b 2 and 13 seem to be major Hev b-allergen specificities (with a recognition ≥50 %), whereas IgE responses to rHev b 1, 3, 5, and 6.01 were present, but in <50 %. 8.3 % of the sera showed sIgE response to cross-reactive carbohydrate determinants (CCDs) [28]. Specific IgE binding to CCDs in vitro may be clinically irrelevant and may not induce cross-linking and histamine release in vivo [25]. However, also genuine latex allergens Hev b 2 and 13 are known to be extensively glycosylated. In contrast to glycosylated nHev b 2, unglycosylated rHev b 2 (produced in E. coli) was not able to bind specific IgE. In these glycosylated allergens, a combined IgE-binding site is conceivable, composed of a peptide and a carbohydrate epitope [28]. Consequently, in cases with positive IgE anti-CCD results in vitro, the clinical relevance must be critically evaluated within the context of the patient’s symptoms [28].
Table 14.3
Protein allergens from natural rubber latex (derived from the sap of the Hevea brasiliensis tree)
Identified allergens | Biochemical name | MW (kDa) | Recombinant protein for in vitro diagnostics commercially available |
|---|---|---|---|
Hev b 1 | Rubber elongation factor | 14 | X |
Hev b 2 | Beta-1,3-glucanase | 34 | |
Hev b 3 | Small rubber particle protein | 24 | X |
Hev b 4 | Lecithinase homologue | 53–55 | |
Hev b 5 | Acidic latex protein | 16 | X |
Hev b 6 | Hevein precursor | 20 | X |
Hev b 7 | Patatin-like protein | 42 | |
Hev b 8 | Profilin | 15 | X
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




