Fig. 11.1
Mechanism of action of imiquimod. Multimodal anti-tumoral activity is achieved via: (1) activation of the NF-kB pathway through TLR-7 and 8 signaling, (2) suppression of negative feedback on inflammatory responses through interaction with the adenosine receptor signaling pathway, and (3) induction of apoptosis through death receptor-dependent and independent mechanisms
In addition to this predominant mode of action, imiquimod has been demonstrated to exert effects independent of TLR-7 and 8 binding. The compound has been shown to inhibit adenylyl cyclase activity, thereby suppressing adenosine receptor signaling pathways. Because these pathways normally inhibit inflammatory reactions, imiquimod augments pro-inflammatory pathways by limiting negative feedback on inflammation. Lastly, imiquimod has also been shown to possess direct pro-apoptotic activity independent of membrane-bound death receptors. Notably, this activity appears to preferentially affect transformed keratinocytes and melanoma cells over their normal counterparts [6, 7, 9].
Efficacy and Recurrence Rates
The first report of using topical imiquimod 5 % cream for the treatment of lentigo maligna was published in 2000. Ahmet et al. successfully treated a large lentigo maligna on the scalp with application of imiquimod over a 7-month period. The use of imiquimod led to gradual clinical clearing of the pigmentation and post-treatment incisional biopsy confirmed the resolution of lentigo maligna. The patient was followed for 9 months without evidence of clinical recurrence, but histopathologic confirmation was not performed [1].
Early case reports and small case series examining the efficacy of topical 5 % imiquimod for lentigo maligna reported clearance rates of up to 100 %, but multiple subsequent larger studies have produced variable results [10–20]. Complete histopathologic response rates have varied from 50 % to 93 %. Comparison between studies has been difficult due to differences in treatment regimen, assessment of outcome, and duration of follow-up. The majority of studies have assessed complete clinical clearance based on targeted biopsies. Among the three studies examining complete post-treatment excision specimens, clearance rates ranged from 53 % to 75 % [10, 11, 13]. A systematic review analyzing data at the individual tumor level calculated overall histologic and clinical clearance rates of 76 % and 78 %, respectively [21, 22].
Recurrence rates have varied from 7.1 % to 50 % with mean follow-up up durations ranging from 6 to 49 months [22]. These rates compare unfavorably to those seen with staged surgical excision and Mohs micrographic surgery, which range from 0 % to 9.7 % and 0 % to 6.25 %, respectively [23]. True recurrence rates with imiquimod may be even higher as the follow-up times in most studies have been shorter than the mean time to recurrence of 3.2 years reported in cases treated with surgery [22, 24]. Limited penetration and consequent incomplete eradiation of malignant cells within adnexa likely account for the higher recurrence rates seen with imiquimod compared to surgical therapy. Prognostic factors that may indicate an increased risk for local recurrence include the total number of melanocytes, number of basal and suprabasal melanocytes, and number of pagetoid spreading melanocytes in the original biopsy specimen [25].
Clinical Treatment Regimen
No standardized application regimen for the use of imiquimod in lentigo maligna exists, and the optimal treatment regimen remains to be determined. Table 11.1 summarizes published treatment regimens for topical imiquimod 5 % cream for lentigo maligna with more than 20 patients. To date, all studies have employed imiquimod 5 % cream; use of the 2.5 % and 3.75 % concentrations has not been studied. A typical regimen is application for 5–7 days per week for 12 weeks with frequency titrated to inflammation [10, 13–15]. Figure 11.2 illustrates a brisk inflammatory response with topical imiquimod with subsequent clinical resolution of the lentigo maligna lesion. A total of greater than 60 applications or a regimen using greater than 5 applications per week has been found to be associated with a higher likelihood of histologic clearance [21]. One study in which subjects were treated for at least 12 weeks and were required to have clinically visible inflammation for at least 10 weeks found a high and sustained clearance rate. All 24 patients in this study experienced clinical and histologic clearance of lentigo maligna and recurrence occurred in only 1 patient after 39 months of follow-up [12]. Topical tazarotene 0.1 % gel may enhance penetration and response, but the clinical significance of this remains to be confirmed in larger studies [26].
Table 11.1
Summary of methodology and results from studies of imiquimod 5 % cream for lentigo maligna enrolling more than 20 patients
Study | Type | No. of tumors | Lesion characteristics | Application regimen | Application area | Assessment of clearance | Complete clearance rate | Follow up period | Recurrence rate |
|---|---|---|---|---|---|---|---|---|---|
Naylor et al. [14] | Prospective | 28 | Majority head (87 %) | Daily × 12 weeks, rest period if intolerable | Lesion + 2 cm margin | Four 2-mm punch biopsies at week 16 | 26/28 (93 %) | 12 months | 0 % |
Cotter et al. [10] | Retrospective | 40 | Majority head/neck (90 %) | 5 days/week × 12 weeks; tazarotene 0.1 % gel nightly added if no erythema at 4 weeks (for 10/40 pts) | Lesion + 2 cm margin | Staged excisions with 2-mm margin | 30/40 (75 %) | 18 months (mean) | 0 % |
Powell et al. [15] | Retrospective | 48 | Face | 3 days/week × 10 weeks; frequency increased to five times per week if no inflammation at 4 weeks | Lesion + 2 cm margin | One 4-mm punch biopsy at week 12 (multiple biopsies if residual pigmentation) | 37/48 (77 %) | 48.6 months (mean) | 0 % |
Ly et al. [13] | Prospective | 38 | Head and neck | 5 days/week × 12 weeks; rest period for excessive inflammation | Lesion + 1 cm margin | Wide local excision by week 16 | 20/38 (53 %) | N/A | N/A |
Alarcon et al. [32] | Prospective | 20 | Face | 5 days/week × 8 weeks; decreased to 3 days/week if excessive inflammation | Lesion + 1 cm margin | Dermoscopy + reflectance confocal microscopy + biopsy at 12 weeks and 12 months after treatment | 15/20 (75 %) | 34 months (mean) | 0 % |
Kirtschig et al. [12] | Prospective | 24 | Face | Daily × 12 weeks titrated to inflammation | Lesion + 1–2 cm margin | Biopsy | 24/24 (100 %) | 39 months (mean) | 4 % |
Kai et al. [45] | Retrospective | 40 | Face | 3 days/week × 8 weeks; increased to 5×/week at week 4 if no inflammation | Biopsy at 3 months after treatment | 27/40 (68 %) | 7.4 months (mean) | 0 % |

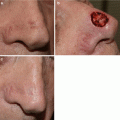
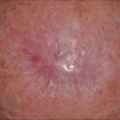
Fig. 11.2
Imiquimod clinical response. (a) Biopsy proven lentigo maligna on right chin. (b) Brisk inflammatory response after 2 months. (c) Clinical resolution of lentigo maligna lesion at 4 months
Assessment of Treatment Response
Optimal technique for assessment of treatment response and outcome is uncertain. Clinical exam is not sufficient, as clinical clearance of pigmentation is known to be an unreliable marker for histologic clearance. Significant residual lentigo maligna can persist histologically despite minimal residual clinical pigmentation as depicted in Fig. 11.3a, b [11]. Conversely, post-treatment hyperpigmentation can be postinflammatory in nature and may not be associated with residual disease [4, 15, 27]. Monitoring for therapeutic response is further complicated by the fact that degree of inflammation does not necessarily correlate with degree of response [26]. While the presence of clinical inflammation tends to portend a histopathologic response, cases in which an inflammatory reaction did not lead to histopathologic clearance or those in which histopathologic clearance was achieved despite lack of clinical inflammation have been described [27]. In Fig. 11.3c, minimal inflammation was noted despite 12 weeks of topical imiquimod treatment for a biopsy proven lentigo maligna. As such, histopathologic examination is necessary to confirm clearance after treatment with topical imiquimod.
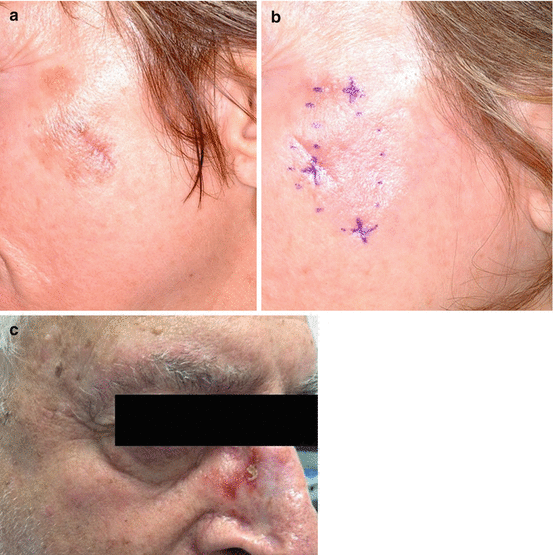

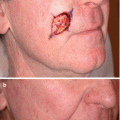
Fig. 11.3
Imiquimod limitations. (a) Broad lesion of lentigo maligna prior to treatment with topical imiquimod. (b) Clinical resolution of pigmented lesion after 12 weeks of topical imiquimod but persistent lentigo maligna histologically at biopsy sites. (c) Minimal inflammation with imiquimod despite 12 weeks of topical treatment
Clinical Monitoring and Follow-Up
There are no official recommendations regarding the appropriate the number, location, and timing of post-treatment biopsies and if biopsies are even indicated. It has been suggested that post-treatment biopsy should be deferred for at least 3 months after completion of therapy, as findings in those performed too soon after treatment may be obscured by an exuberant interface dermatitis reflecting continued inflammatory activity from treatment effect [15]. However, it is unknown how best to identify the correct site or sites for biopsy in order to avoid sampling error that may miss foci of residual disease and lead to false negative results. Appropriate monitoring for recurrence similarly remains to be established, as long-term follow-up studies are not yet available. Close, regular clinical follow-up with a high index of suspicion for any concerning areas is imperative.
Several modalities for improving selection of biopsy site and detection of residual or recurrent disease have been proposed. The most simple of these is use of the Wood’s lamp, which has been shown to be helpful in the delineation of clinical borders of lentigo maligna by enhancing the appearance of pigment in the skin [28]. Dermoscopy and reflectance confocal microscopy, either alone or in conjunction, have also been studied for this use. Four dermoscopic features—(1) asymmetric pigmented follicular openings, (2) dark brown or black rhomboidal structures, (3) slate-grey dots, and (4) slate-grey globules—were initially described as correlating highly with lentigo maligna on the face [29, 30]. A subsequently observed finding of very fine, dust-like brown dots, thought to correspond to pagetoid cells migrating through the epidermis, has also been highly correlated to the presence of lentigo maligna [31]. Reflectance confocal microscopy, which allows for in vivo optical sectioning of the skin to a depth of 200 μm, has demonstrated superiority over dermoscopy for delineation of margins with lentigo maligna. Based on the identification of features comprising a lentigo maligna “score,” reflectance confocal microscopy appears to provide greater sensitivity and specificity for detection of recurrent lentigo maligna over dermoscopy [31–35].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree