CHAPTER 25 Revision Total Ankle Replacement
MANAGEMENT OF FRACTURES
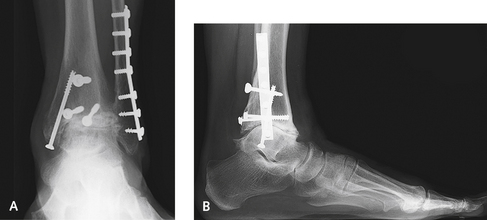
The medial malleolus is at risk for fracture regardless of the type of implant used, but more so with the Agility prosthesis, for which a vertical cut is made in the malleolus. For malleolus-sparing procedures, the risk for fracture is nevertheless still present, and in any patient with osteopenia, I protect the malleolus with prophylactic pinning. I insert two Kirschner wires (K-wires), and if there appears to be higher risk for malleolar fracture, I use cannulated screws over the guide pins. The medial malleolus is at particular risk for fracture in the patient who has sustained a previous fracture, and in whom the screws are still present and are in the path of the prosthesis (Figure 25-1). If the implant can be inserted with the screws left in, this is preferable, but if hardware removal is necessary, then multiple guide pins or K-wires should be inserted to protect the malleolus.
If fractured, the medial malleolus must be fixed using small cannulated screws or a tension band during the same procedure. The replacement is at tremendous risk for subsequent disaster unless the malleolar fracture is treated accurately. It is essential to make sure that there is no deformity of the foot causing subsequent stress of the malleolus, which would be the source of a delayed malleolar fracture. This pathogenic mechanism is more common with a valgus foot deformity, with stress on the deltoid and medial malleolus. It may be necessary to sacrifice some range of motion for the need for stability and to immobilize the ankle postoperatively. Screw fixation should be used as a preventive measure in patients with severe osteopenia, and a fracture that occurs intraoperatively should always be repaired with screws (Figures 25-2 and 25-3).
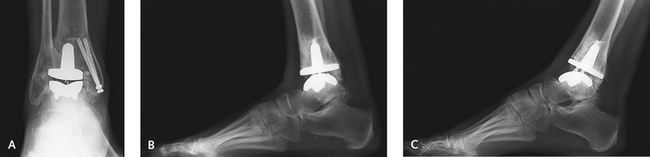
In general, I am not as concerned for the fractured fibula, because these fractures are easy to fix. Once again, if a fracture occurs, it must be surgically stabilized and the anatomy restored. Fractures may occur inadvertently with the cut on the tibia, regardless of the type of prosthesis (Figure 25-4). Severe valgus instability with lateral shift and tilt of the talus may result. Although all implant procedures carry a risk of fracture, the risk is higher with use of the Agility prosthesis, which emphasizes the enhanced stability of the tibial component by syndesmotic fusion. A point to remember is that the fibula lies far posteriorly relative to the tibia and is at risk for fracture when the tibial cut is made. Another source of fracture with the Agility prosthesis is overcompression of the fibula during syndesmotic screw fixation. This too presents a potentially serious problem: overcompressing the fibula can cause valgus failure of the prosthesis. Because a plate is routinely used on the fibula to enhance syndesmosis fusion, the complication of fibula fracture is not as important, but the alignment of the fibula must be maintained.
MANAGEMENT OF WOUND HEALING PROBLEMS
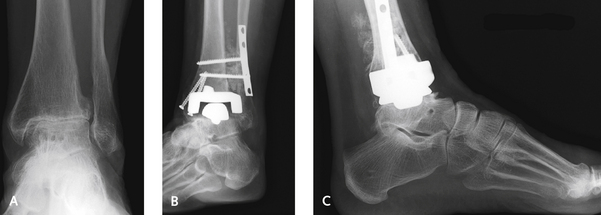
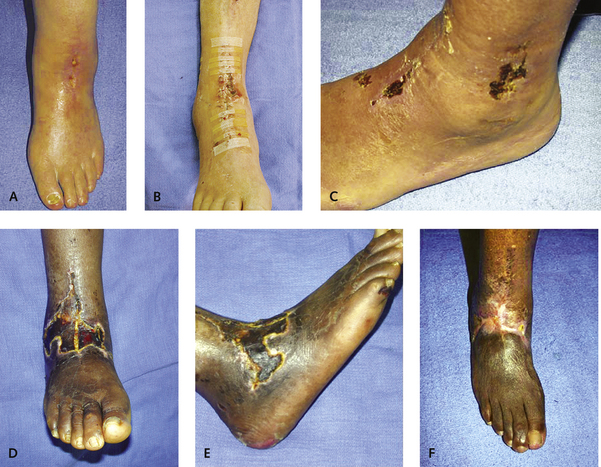
A minor dehiscence of the incision is not uncommon postoperatively. The very best treatment of this complication is use of simple topical antibiotic agents, dressing changes, and topical care of the skin (Figure 25-5). If a superficial dehiscence occurs, it is treated with daily changes of wet-to-dry saline dressings. I like to use a wound vacuum-assisted closure (VAC) device whenever there is drainage with breakdown of the incision. Deeper wound breakdowns are treated with silver sulfadiazine (Silvadene) dressings. If an eschar develops over an area of the incision, it is best left alone. The wound is not debrided and is kept as dry as possible (see Figure 25-5, C–F). The size of the eschar does not seem to be of any consequence, and over time and in the absence of infection, these dry eschars demonstrate quite a remarkable potential for healing. With more extensive wound dehiscence and exposure of the anterior tibial tendon, the use of a wound VAC device works well; in this setting, formal debridement of the wound and tendon is not necessary, because the VAC device may promote granulation and complete coverage of the tendon, without increased risk of infection. If deeper breakdown does occur, and the component is potentially exposed, coverage with a free flap usually is required to prevent infection and failure of the joint replacement. Removal of the prosthesis should not be necessary if coverage is adequate. I have no experience with acute infection of the joint; if this occurs, however, exchange of the polyethylene (“poly”) insert plus irrigation as is performed for other joints would be prudent.
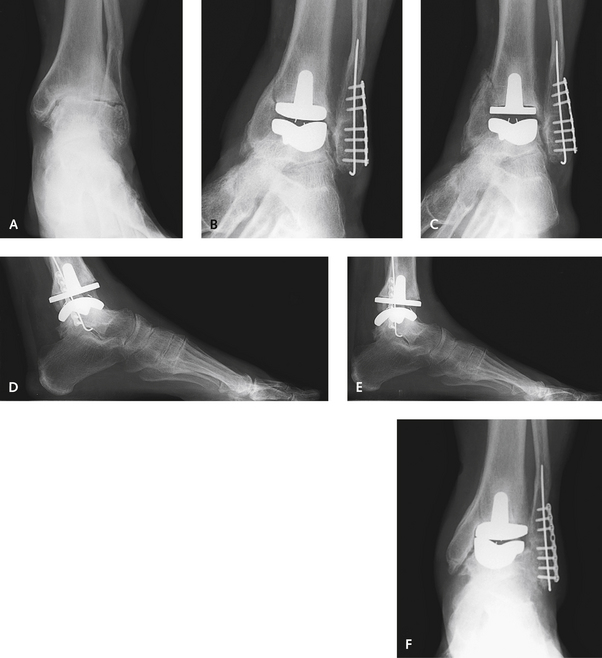
More advanced infection should be treated with removal of the components, insertion of an antibiotic spacer, and administration of intravenous antibiotics. Although antibiotic beads can be made to fill the gap, a solid cement spacer acts to keep the joint space open, distracted at the correct soft tissue tension, and more stable. I permit patients to walk on the ankle as soon as the skin incision has healed after treatment of infection, without concern for any further bone loss or subsidence. After 6 weeks, an aspiration of the joint is performed, and if results of aspirate culture are negative, 4 weeks later the prosthesis is reimplanted. Before the surgery, I take a biopsy specimen of the synovium, and if the cell count is greater than 6 white blood cells per high-power field, the joint is debrided and another antibiotic cement spacer is inserted until the joint is proved to be infection-free. It is curious how well some patients tolerate the cement spacer. One of my patients who underwent an ankle replacement 5 years earlier developed a deep infection shortly after surgery performed through a lateral incision. The prosthesis was removed, and an antibiotic-impregnated cement spacer was inserted. During his subsequent follow-up care, the patient commented that this was the best that his ankle had felt in years, and he did not desire any further treatment. At the 5-year follow-up evaluation, no evidence of subsidence or erosion of bone was found, and the patient retained approximately 15 degrees of motion (Figure 25-6).
CYST FORMATION
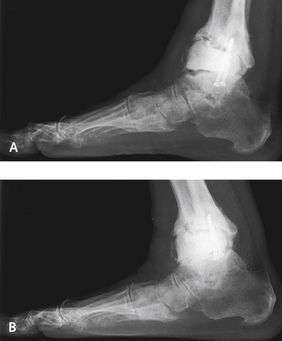
If the patient presents with an asymptomatic cyst on the plain radiograph, obtaining a CT scan is helpful, but the surgeon must be prepared to perform a revision if the size of the cyst is progressively enlarging (Figure 25-7). The CT findings are always far worse than expected, and as noted, these cysts can be quite massive.
“Expansile” osteolysis resulting from polyethylene wear debris tends to progress, with consequent destabilization of the implant, leading to loosening and, ultimately, periprosthetic fracture. So, despite the total lack of symptoms in some patients, I try to encourage them to understand the cause, with its implications for the need for a timely revision to prevent catastrophic mechanical failure and to preserve and supplement the remaining bone support. A real therapeutic dilemma arises if the patient is asymptomatic, with good range of motion, and plain radiographs and CT scans show an apparently stable prosthesis but large osteolytic cysts. It is hard to convince a patient to undergo a revision when the joint is entirely asymptomatic. Serial investigations and close follow-up examination may be acceptable to help the patient decide when a revision or bone grafting is needed.
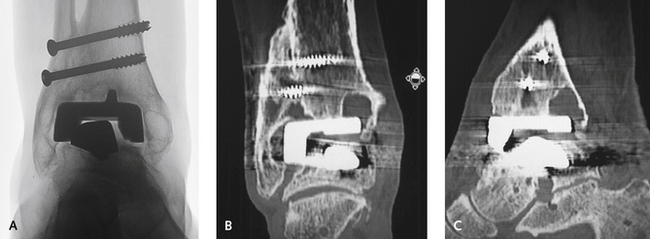
Once a decision for surgical management of the cysts has been made, it generally is necessary to revise the prosthesis, or at the very least, the “poly” liner. Generally, there is some minor abnormality with alignment causing the abnormal poly insert wear. The cysts often are far deeper than is apparent on plain radiographs, and a large curette is used to remove the lining of the cyst without perforating the bone. I pack the cyst with a mixture of cancellous allograft chips and an iliac crest aspirate concentrate. The cancellous graft is packed in firmly, particularly when the bone loss is at the margin of the tibial component (Figure 25-8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree