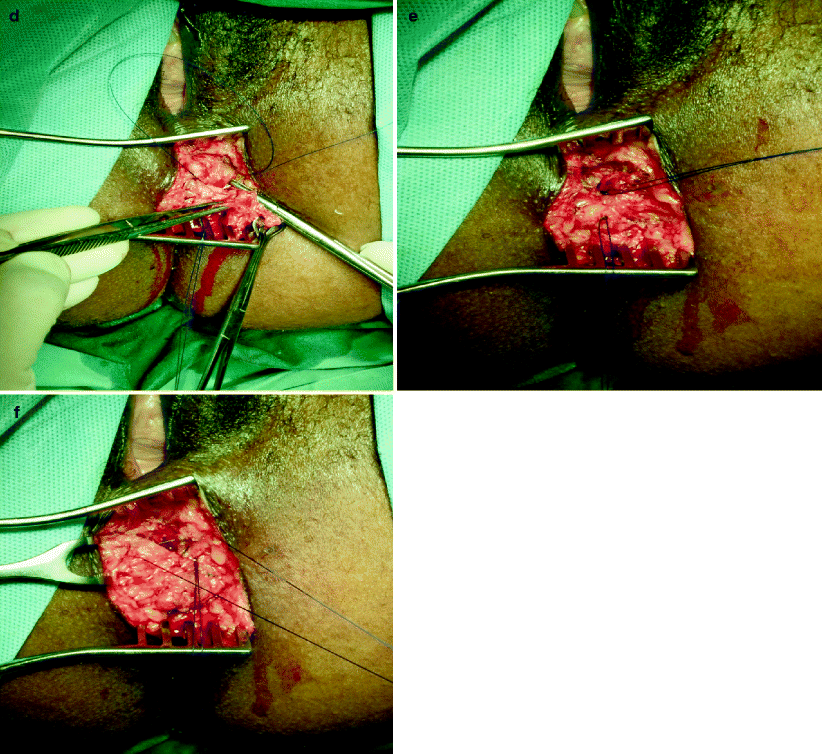
Fig. 29.1
(a) Transperineal dissection of attenuated external anal sphincter (arrow). (b) Atrophic prior repair held in Babcock forceps. (c) External anal sphincter divided and mobilized for repeat overlapping repair. (d) Overlapping external anal sphincteroplasty. (e) Completed overlapping external anal sphincteroplasty. (f) Concomitant anterior levatorplasty (suture untied) (Photos courtesy of A. P. Zbar)
Other Options
When sphincteroplasty fails, other treatment options for fecal incontinence include sacral nerve stimulation, the implantation of an artificial anal sphincter, postanal repair, dynamic graciloplasty, or colostomy. Sacral nerve stimulation has been used for the treatment of fecal incontinence for more than a decade, with good results in patients with or without a sphincter defect [20–23]. The available long-term results (up to 7 years) are satisfactory [24, 25]; however, this modality it is still not approved as a primary indication for fecal incontinence in the US, where it is limited to patients with combined fecal and urinary incontinence. The success of sacral neuromodulation in Europe in patients with a demonstrable external anal sphincter defect has raised questions concerning the role of formal sphincteroplasty. Limited data have shown most such patients progressing toward permanent implantation after successful temporary stimulation [26] along with a significant improvement in quality of life scoring because it affected lifestyle, coping behavior, depression, and self-perception scoring and embarrassment scores [27]. Although all patients in the study by Brouwer and Duthie [27] from Hull, England, improved their continence score, whereas patients with a sphincter defect seen on endoanal ultrasonography, those with a pudendal neuropathy, and those who had undergone a previous sphincter repair reverted to their pretreatment continence scores over a follow-up of 37 months (range, 15–41 months).
Similar data has been shown recently by Ratto and colleagues [28] from Rome. Their small randomized trial comparing those undergoing adequate sphincteroplasty (as confirmed by endoanal ultrasound) with those with an external anal sphincter defect undergoing sacral neuromodulation showed no difference in results after a median follow-up of 60 months. The durable success of sacral neuromodulation and its indications within subgroups of patients who present with fecal incontinence and a demonstrable external anal sphincter defect will have a significant impact on the role of the simpler formal sphincteroplasty operation for this condition both in the primary and the reoperative setting. At present, the relative lack of availability of sacral nerve stimulation and its confinement to specific centers, the lack of wider approval for its use in the US, and its cost somewhat preclude its more extended use in this clinical circumstance. The current initial ability of repeat sphincteroplasty to improve most patients (despite gradual deterioration of functional results over time) may still make it an acceptable treatment in smaller centers when performed by experienced coloproctologists. Sustainably good long-term results of sacral nerve stimulation and a better understanding of subgroups and their evaluation when its use results in worse longer-term outcomes will resolve this important issue and decide on the viability of sphincteroplasty as an acceptable operation when there is a sphincter defect [29].
The artificial anal sphincter is still available and has acceptable results, although the use of this technique is somewhat waning because even in expert hands the infection rate is reported at 33% [30, 31]. The reoperation rate is moderately high, in many cases leading to device failure or extrusion, and it cannot specifically be advocated for use in patients presenting with recurrent fecal incontinence after a failed sphincteroplasty. Postanal repair is indicated for neuropathic incontinence and is not popular because its earlier results could not be duplicated. At best, only about 30% of patients report sustained improvement [32–35], although some long- term studies have shown good functional results [36, 37]. It is unclear whether formal pelvic floor repair (i.e., overlapping anterior anal sphincteroplasty plus postanal repair) offers any significant functional advantage for cases of recurrent so-called neurogenic incontinence [38]. In those cases where the quality of the external anal sphincter is poor, precluding mobilization, an alternative may include muscle imbrications (without division) combined with other pelvic floor reparative procedures, although there are relatively little data from this patient group [39].
Dynamic graciloplasty (covered in Chap. 30) is a complex procedure requiring specific expertise, which is expensive and often associated with considerable morbidity and a moderate operative revision rate [40–46]. The stimulator required to change the muscle fibers to a slow-twitch muscle is currently unavailable for clinical use in the US. Injectable bulking agents using a range of bioaugmentable agents designed to supplement the disrupted internal anal sphincter have been shown to be effective in some studies but are not presently approved in the US for the specific treatment of fecal incontinence [47–50]. This topic also is covered in Chap. 33. Other potential alternatives being investigated for this cohort of recurrent patients include the use of a transobturator rectal sling (which is undergoing trials in the US) [51] and some novel animal work using muscle progenitor cell autograft transplantation, which has shown accelerated myofiber repair in the damaged sphincters of rabbits along with enhanced sphincteric electromyographic activity [52].
Conclusion
Repeat sphincterplasty is a viable option for patients with fecal incontinence. The rate of acceptance of repeat sphincter repair is the same as that after a primary repair and, hence, should be considered for selected patients with failed primary repairs. Coloproctologists can advise their patients (despite widespread available data) that most are likely to be improved, although there will be a natural deterioration in efficacy over time, similar to that experienced with a primary sphincter repair. Although many patients are not what they might consider “normal” (continence remains significantly diminished when compared with controls), this continence improvement permits a correlative improvement in social and sexual functioning [53]. Its specific role in the era of sacral neuromodulation, even when there is a demonstrable external anal sphincter defect, is unclear, but it would seem to be the cheapest and easiest option in the treatment of recurrent severe fecal incontinence when nerve stimulation is either unavailable or unapproved.
References
1.
2.
3.
4.
5.
6.
7.
8.









