Fig. 36.1
Delayed mucosal advancement anoplasty with internal and external anal sphincter repair after preliminary seton placement. (a) Fistula dissected at the internal opening. (b) Internal anal sphincter repair. (c) Mucosal advancement closure (Courtesy Professor A.P. Zbar)
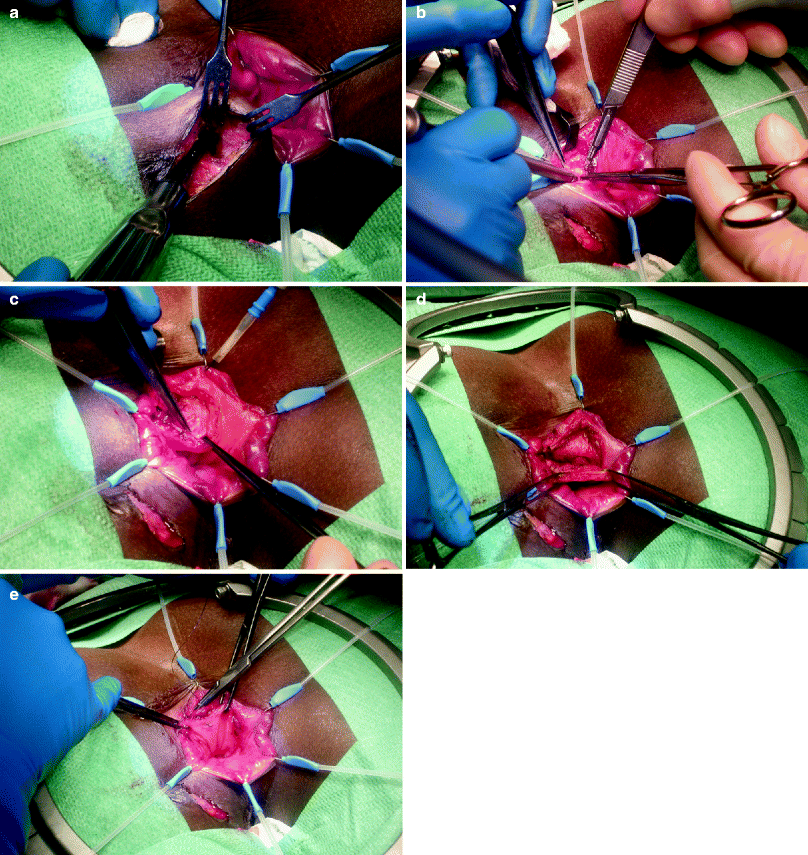
Fig. 36.2
Transanal advancement flap repair. (a) The external part of the fistula is cored out until the intersphincteric space is reached. (b) The internal opening is excised and the intersphinteric space is debrided. The overlying anoderm is excised. A flap consisting of mucosa, submucosa, and some superficial fibers of the internal anal sphincter is raised. (c) The flap is raised from the level of the dentate line and mobilized over a distance of 4–6 cm cephalad. (d) Care is taken to create a broad-based flap that is well vascularized. (e) The flap is advanced and sutured to the neodentate line with absorbable sutures
Technical Considerations
Considerable debate exists concerning the thickness and consistency of the advancement flap repair. Most authors [5, 22, 31–37] have used a flap consisting of mucosa, submucosa, and some of the most superficial fibers of the internal anal sphincter. This technique allows for a relatively bloodless dissection and yields a substantial flap with enough elasticity to be advanced over the internal opening. However, advancement flaps consisting of only mucosa and submucosa also have been described [38], as have full-thickness flaps consisting of the full thickness of the rectal wall [4, 39–41]. Some studies have been performed comparing the use of full-thickness flaps with flaps consisting of only mucosa and submucosa [40–42]. A small, retrospective study suggested improved healing after the use of full-thickness flaps without additional impairment of continence [40]. Another recent small, randomized study by Khafagy et al. [41] also showed better results for patients in whom a full-thickness flap was created; however, this group found a higher incidence of impaired continence in these patients. Roig et al. [42] performed a meticulous retrospective analysis of 70 patients undergoing endorectal flap repair and found no differences in healing, although they did report a trend toward poorer functional results after the use of full-thickness flaps. Even though there is a small amount of evidence, it would seem prudent to add some superficial sphincter fibers to the advancement flap to strengthen the flap coverage. It is noteworthy that most large series that have been published and have used this technique have utilized this partial-thickness flap incorporating the superficial sphincter.
Contributing Factors: Smoking
Several factors that might affect the outcome of transanal advancement flap repair have been analyzed. Several authors found a detrimental effect of the use of tobacco [43, 44]; however, this effect could not be confirmed by others [4, 37]. In a previous study designed to assess rectal mucosal blood flow before and after flap repair in smoking and nonsmoking patients, Zimmerman et al. [32] observed a significant decrease in rectal mucosal blood flow after creation of the flap. Furthermore, rectal mucosal blood flow was found to be significantly lower in smoking patients both before and after transanal advancement flap repair; however, no correlation was found between blood flow and the outcome of transanal advancement flap repair [45]. This observation indicates that diminished rectal mucosal blood flow does not have a detrimental effect on transanal advancement flap repair. However, several investigators found a decreased healing rate after advancement flap repair in patients who smoke cigarettes, and it would seem sensible to counsel patients about this finding. Smoking cessation seems prudent in patients with recurring fistulae for whom repair will be attempted with an advancement flap. Ellis and Clark [44] found a negative effect of smoking on the outcome of mucosal advancement but not on the outcome of anocutaneous advancement flap repairs; therefore, this type of repair possibly can also be considered in such cases.
Contributing Factors: Preoperative Seton Drainage
Some authors advocate preoperative seton drainage because it allows drainage of the fistulous tract, thereby reducing inflammatory activity, reducing the chance of interim abscess formation, and resolving secondary tracts [2, 46, 47]. Although several authors [2, 22, 46, 47] found that preoperative seton drainage improves healing after transanal advancement flap repair, this beneficial effect could not be confirmed by others [43, 48, 49]. Moreover, the reported series were rather small and study populations often were heterogenous, including patients with rectovaginal fistulae and those with fistulae caused by Crohn’s disease. In conclusion, there seems to be no convincing evidence for the use of seton drainage before transanal advancement flap repair even though it has been reported to be a specific part of surgical algorithms for staged repair [50, 51]. However, it is possible that seton drainage was used in more difficult cases and that the preoperative eradication of active sepsis may have allowed the rate of healing of complex fistulae to approach that of more straightforward cases. Moreover, we acknowledge the importance of eradicating all active perianal sepsis before attempting any repair of a complex perianal fistula. For this purpose, a seton drain can be a useful tool, and preoperative seton drainage is still an important part of the surgical armamentarium in surgery for perianal fistulae, as opposed to the use of a seton for definitive cutting purposes, which may be associated with a significant and under-recognized incidence of functional continence disturbance [52, 53].
Transanal Advancement Flap Repair for Recurrent Fistulae
Lindsey and colleagues [49] have reported that the efficacy of a repeat transanal advancement flap repair is limited because the scarring associated with a failed advancement flap compromises the changes of success with subsequent flaps. However, this statement is not supported by the literature. In a study conducted by Kodner et al. [5], nine patients, in whom the initial flap repair failed, underwent a second operation, which was successful in all patients. In a further study conducted by Mizrahi and colleagues [4], 12 patients underwent repeat surgery because of initial flap failure, 8 of whom (67%) healed. Several large series [4, 22, 38, 43, 54] have investigated the use of transanal advancement flap repair for recurrent fistulae. Our group [43, 54] found that prior operations did not influence the healing rate of this technique. We believe this is a strong argument in favor of utilizing this technique where possible in patients with recurring complex fistulae.
Functional Considerations
Recently, several large series [4, 22, 38, 43, 54] have investigated the use of transanal advancement flap repair for recurrent fistulae; unfortunately, the impact on fecal continence was not properly assessed in any of these studies. It has been suggested that transanal advancement flap repair may result in impaired fecal continence. The reported incidence of this side effect varies between 8 and 35 % [31, 38]. Any decrease in fecal continence is usually minor (soiling or incontinence for flatus); according to some authors, inclusion of internal anal sphincter fibers contributes to the impairment of continence. On the basis of this assumption, it might be possible that a second flap repair results in further deterioration of fecal incontinence. A recent study of 87 patients by Mitalas et al. [54] showed that a repeat transanal advancement flap repair has a healing rate comparable to the first attempt without significant deterioration in postoperative fecal continence.
Transanal Advancement Flap Repair in Patients with Crohn’s Disease
The use of mucosal advancement flap repair of perianal fistulae in patients with Crohn’s disease has been investigated by several authors. Initially, reticence with using this technique was advocated because reported healing rates varied widely, between 33 and 83 % [39, 55]. Furthermore, only small groups of patients were reported. Makowiec et al. [56] reported a relatively large series consisting of 36 operations in 32 patients with Crohn’s disease who were undergoing mucosal advancement flap repair. Even though short-term success seems to be acceptable (only four immediate failures were reported), fistula recurrence was reported in 17 patients, bringing the cumulative probability of overall fistula recurrence to 66 %. Functional results were, however, reported to be good. These results are remarkably similar to those of Mizrahi et al. [4] and Sonoda et al. [22], who reported healing rates of 43 and 50 %, respectively. Unfortunately, neither of these author groups presented a detailed objective analysis of the functional results. It is noteworthy that both groups found that the presence of Crohn’s disease was a significant negative predictor of success.
A recent systematic review on this subject by Soltani and Kaiser [57] computed the weighted average healing rate of mucosal advancement flap repair of perianal fistulae in patients with Crohn’s disease to be around 64 %. Unfortunately, they based their calculation of the weighted average healing rate on only immediate healing rates. Two relatively large studies [58, 59] that were included in this calculation reported healing rates of 75 and 71 %, respectively. In these studies, further recurrences were reported, bringing the ultimate success rates down to 44 and 50 %, respectively. Furthermore, small series (fewer than ten patients) seem to be over-represented in this calculation.
In conclusion, the healing rate of mucosal advancement flap repair of perianal fistulae in patients with Crohn’s disease is low. However, healing is possible and functional results seem to be relatively good, although few detailed data are available. Therefore, this procedure can be considered in selected patients who are willing to accept a relatively low chance of a successful outcome.
Fistulectomy with Immediate Sphincter Repair
An under-utilized technique that was originally described by the group at St. Mark’s Hospital in London in 1993 [58] and later modified by Athanasiadis et al. [60] is fistulectomy with immediate sphincter repair. Some promising results with this technique have been published, although it is likely that the technical challenge in performing this technique correctly is the reason that it has not found widespread acceptance.
Surgical Technique
The internal opening and the intersphincteric part of the fistula tract are completely excised up to the intersphincteric plane. The intersphincteric area is curetted (with evacuation of the cryptoglandular focus of infection), and then a wide elliptical incision around the external opening is made up to the locus at which the tract traverses the external sphincter. The fistula tract with surrounding skin and fat tissue are removed en bloc. Intra-anal closure then is performed, and the excised internal fistula ostium is closed using several interrupted sutures, the first row of which is approximated in the internal sphincter. A second row of sutures then is placed, grasping parts of the internus fibers, submucosa, and mucosa. The circular defect in the external sphincter is finally coapted using a perianal approach with interrupted sutures.
Technical Considerations
The scarce reports that are available are all of relatively good quality [42, 58, 60, 61]. Incontinence was measured adequately and the surgical technique was described in detail. The original report describes an intersphincteric approach, although this approach was abandoned by other authors. Even though this approach was the inspiration for the ligation of intersphincteric fistula tract (LIFT) technique that was developed later [62], it has been abandoned by other surgeons. At first sight, it seems illogical to add a wound over the intersphincteric plane when the fistula can be approached intra- and perianally. However, in light of current developments, this statement can be questioned. An intersphincteric approach possibly allows better eradication of intersphincteric sepsis. There is no evidence for this in the few reports of fistulectomy with immediate sphincter repair technique, and the reported healing rate in the initial report is fairly low when compared to other reports.
Both Matos et al. [58] and Perez et al. [61] closed the external wound. In the latter group’s report, an extremely high incidence (71 %) of wound complications was reported. Even though Matos et al. do not report the incidence of postoperative wound complications, it is possible that these complications are part of the reason their success rate is lower than that of other authors. When the external wound was left open, the incidence of postoperative wound complications was still relatively high: 15 % in one report [60] and 19 % in another [42].
Several authors compared this technique with the use of endorectal advancement flap repair [42, 63]. Neither group found a significant difference in recurrence rates, but Roig et al. [42] reported a statistically significantly higher incidence of impairment of continence after endorectal advancement flap repair, especially after creation of a full-thickness flap. Experienced authors state that not all transsphincteric fistulae are suitable for this type of repair [60]. Some patients generally exhibit scarring around the internal fistula opening and in the anal canal region. In these cases, the surrounding tissues are affixed and less elastic as a consequence of earlier operations and are not suitable for a direct closure. Ideal candidates for a direct approximation of muscular structures are women and men with a relatively short and elastic anal canal with a dorsal internal fistula opening. An alternative is the staged technique of Zbar et al. [64], the internal anal sphincter is repaired in accordance with the technique described by Athanasiadis et al. [60] after preliminary seton drainage, but the seton is rerouted intersphincterically for delayed repair. The clinical results of Zbar and colleagues were superior in terms of recurrence rate to those of Athanasiadis et al. in a small prospective, randomized trial, and the resting manometry was superior in the group that underwent primary internal anal sphincter repair, although this did not reach statistical significance [64].
Use of Fistulectomy with Immediate Sphincter Repair in Patients with Crohn’s Disease
Limited data are available concerning the use of fistulectomy with immediate sphincter repair in patients with fistulae that are caused by Crohn’s disease. Most studies exclude patients with Crohn’s disease. Matos and colleagues [58] described only five patients suffering from inflammatory bowel disease. The operation failed in all of these patients and as a consequence, no meaningful conclusions can be drawn from these limited data.
General Considerations
The reported healing rate after the use of this technique is relatively high (between 69 and 93 %). Furthermore, there are only small differences between healing rates reported by different authors. Fecal continence was assessed by all authors; even though only minor incontinence is reported after this procedure, the incidence of decreased fecal continence is moderate, varying between 6 and 33 %. A logical apprehension when performing this technique is the risk of postoperative sphincteric disruption and thus of fecal incontinence. Perez et al. [65] were the only ones to perform an overlapping sphincter repair as part of this procedure. Even though they report a high healing rate (93 %), there does not seem to be an obvious advantage in performing an overlapping sphincter repair as opposed to an appositional sphincter repair. It is noteworthy that the use of fistulectomy with immediate sphincter repair does not seem to reduce anal canal pressures, as has been observed by several groups [42, 65].
Limited data exist on the use of fistulectomy with immediate sphincter repair in patients with recurrent fistulae. Few authors include data concerning prior operations in their reports. Athanasiadis et al. [60] include a fairly large group of 51 patients who have undergone prior surgical attempts at repair of their fistula. Interestingly, they found no difference in healing rate. Similar results were reported by Roig and colleagues [42], who concluded that recurrent fistulae were the main indication for the use of this technique.
Anodermal (or Anocutaneous) Advancement Flap
Anocutaneous advancement flaps were introduced in the treatment of complex perianal fistulae by del Pino et al. [66]. They reported a small number of patients with promising results. According to these authors, this procedure does not result in anatomic alteration of the anal canal, so all other operative choices are subsequently still feasible. Others have reported similar results; however, the technique has not found widespread acceptance.
Surgical Technique
The internal opening of the fistula is exposed and the crypt-bearing tissue around the internal opening as well as the overlying anodermis is excised. The fistulous tract, running from the external opening to the external anal sphincter, is excised. The tract running through the sphincters is excised or curetted. The defect in the internal anal sphincter is closed with absorbable sutures. An (inverted) U-shaped flap including the perianal skin and fat is created, taking care not to undermine the flap to prevent ischemia. The base of the flap should be approximately twice the width of its apex. The flap then is advanced and sutured to the mucosa and the underlying internal anal sphincter in a single layer, proximal to the closed internal opening, using interrupted, absorbable sutures. The perianal wound is left open.
Technical Considerations
There is considerable variability in the surgical technique as utilized by different authors. The initial report described a pear-shaped flap that was advanced into the anus, after which the external defect was left open [66, 67]. Others describe the use of a diamond-shaped flap, allowing the external defect to be closed [68]. Other variations that were reported, with which it was possible to close the external wound, are the V-Y anoplasty [69, 70] and the use of Burrows triangles [71]. We have used an inverted U-shaped flap [72, 73] and, to minimize the risk of wound infection, we leave the external defect open. Amin and colleagues [69] adapted their technique by leaving open part of the V-Y reconstruction to cope with this problem. It is hard to draw meaningful conclusions regarding this approach because wound-related problems are not specifically reported. However, it would seem that authors who utilize techniques that enable closure of the donor site [68–71] report a higher incidence of wound-related problems than authors who leave the donor site open [66, 67, 73].
Several authors report the possibility of readvancing the cutaneous flap in cases of failure [67, 69]. Nelson et al. [67] performed a redo anocutaneous advancement in 13 patients. This repair was successful in nine patients (69 %). These authors conclude that one of the main advantages of the anocutaneous flap is the possibility of reoperation and repetition, with a high probability of success. Amin et al. [69] reach the same conclusion, even though they performed redo anocutaneous advancement flap in only two patients.
Use of Anocutaneous Flap Repair in Patients with Crohn’s Disease
Limited data are available concerning the outcomes of this technique in fistulae that are related to Crohn’s disease. Nelson and colleagues [67] included 17 patients with Crohn’s disease in their report on this technique, and they found only two recurrences in this group of patients. Because this is the only substantial report of the use of this technique in patients with Crohn’s disease, it is not possible to reach any meaningful conclusion.
General Considerations
The use of this type of repair for fistulae that failed to heal after other types of surgery also has been described by several authors [67, 70, 71, 73]. In a small series reported by members of our group [73], a difference was found in the healing rate between patients who had undergone no or only one previous attempt at repair and patients who had undergone two or more previous attempts at repair. This difference also was noted by other authors, without reaching statistical significance [67, 70].
Conclusions
The anodermal advancement flap is a relatively easy technique that seems to be especially promising for primary fistulae. Several authors have published good results for primary, non-Crohn’s disease–related fistulae using this type of repair. For recurrent fistulae, this technique seems to be less suitable. If this technique is used, it seems prudent to leave the external wound open.
The Anal Fistula Plug
In recent years, the Surgisis anal fistula plug (Cook Surgical Inc., Bloomington, IN) has been developed as an alternative to traditional fistula surgery. This plug consists of an extracellular matrix derived from the submucosa of porcine small intestine. This matrix supports remodeling of the host tissue, resulting in closure of the fistulous tract, and it does not encapsulate when implanted. According to some authors, this minimally invasive technique provides a simple and safe option in the treatment of fistulae. Initially, the reported healing rates were high – up to 87 % [74, 75]. More recently, however, less promising results have been reported, with healing rates dropping to 41 % [76], 24 % [77], and 14 % [78] in more complex fistulae.
Surgical Technique
After irrigating the fistulous tract with hydrogen peroxide, the conical device is placed by drawing it through the fistulous tract. It then is cut to fit and is secured in the internal opening using a figure-eight suture incorporating it with the mucosa to close the internal opening.
Considerations
Ellis and Clark [44] were the first to compare anal fistula plug closure with transanal advancement flap repair. Their retrospective analysis revealed that the fistula recurred in 33 % of the patients after flap repair and in 12 % of the patients after plug repair. Although this difference seems to be in favor of the anal fistula plug, it did not reach statistical significance. A similar finding was reported by Chung and coworkers [79]. Recently, two retrospective studies revealed that flap repair provides a significantly higher healing rate than the use of an anal fistula plug [80, 81]. This observation is supported by the outcome of a randomized, controlled trial [82].
Conclusions
On the basis of these data, flap repair seems to be more favorable than plug repair. However, the anal fistula plug might provide a safe and easy alternative for a selected group of patients with simple low transsphincteric fistulae. The relatively high cost of the plug is another drawback, and at present, the fistula plug does not seem to be an attractive option for recurrent complex fistulae.
LIFT Procedure
A relative newcomer is the LIFT procedure (Fig. 36.3a–e), which recently was described by Rojanasakul et al. [62], although aspects of this approach had been reported previously [60, 64]. Relatively few data are available on this technique; however, the results seem to be promising.
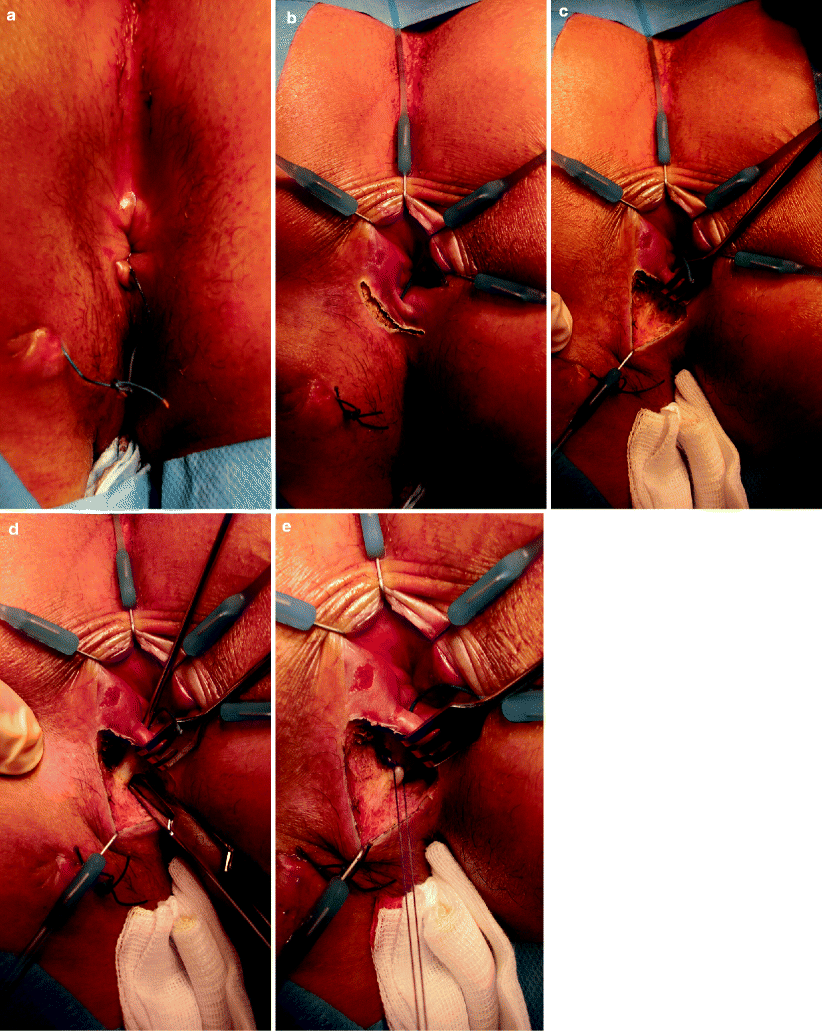

Fig. 36.3
Ligation of intersphincteric fistula tract procedure. (a) A seton is placed in the fistula to induce fibrosis. (b) A small incision is made in the intersphincteric groove. (c) The intersphincteric space is developed. (d) The intersphincteric part of the fistula is identified. (e) The intersphincteric part of the fistula is carefully ligated and subsequently divided
Surgical Technique
A probe is maneuvered through the fistula tract. With the probe in place, the skin over the intersphincteric groove is marked. A curvilinear incision is made in the intersphincteric groove and the tract is identified in this space with the probe in situ, facilitating identification of the tract. Care is taken not to divide any sphincter. Once the tract is dissected free, it is encircled and the probe can be removed. Next, each side of the tract is suture ligated, and the tract is divided. The external opening is left open and the external part of the tract is curetted. Some gentle curetting can be done through the internal opening as well, and the internal opening then is closed. Finally, the skin incision is closed with interrupted absorbable sutures.
Technical Considerations
There is some variability in the manner in which the fistula tract is ligated. Bleier et al. [83] ligated each side of the divided tract after division, whereas in the original report [62], the external part of the fistula was excised. Shanwani et al. [84] also cored out the external part of the fistula.
Considerations
Recurrence of fistula-in-ano is thought to be due to fecal material entering the internal opening (because of a pressure gradient between the anal canal and the perianal skin). The LIFT technique prevents the entry of fecal material into the fistula tract and eliminates the formation of a septic nidus in the intersphincteric space, permitting healing of the fistula-in-ano. The advantages of the technique may include preservation of the anal sphincter, minimal tissue injury, and shorter healing time in a procedure that is relatively easy to perform. The procedure can be used in patients who have recurrences from almost any other previous technique. The healing rates that have been reported for this technique are relatively high, between 57 and 99 %.
The majority of patients that were operated on by Bleier et al. [83] had undergone previous attempts at repair of their fistula (74 %), and possibly this is the reason why the reported healing rate is relatively low. In their report, however, they found no impairment of continence, although they did not use a detailed questionnaire or other validated tool to assess continence.
Use of the LIFT Technique in Patients with Crohn’s Disease
Even though there could be a theoretical advantage to the utilization of this technique in patients with Crohn’s disease, at present no data are available.
Conclusions
This new technique for fistula-in-ano surgery aimed at total anal sphincter preservation shows encouraging early results that seem to compare well with those found with other sphincter-saving procedures. Randomized, controlled trials with long-term follow-up seem to be both worthwhile and necessary to define the exact place of this technique in the armamentarium of fistula surgery.









