Type of lesions
%
Method of localization
Microcalcification
33.5
STEREOTACTIC
Radial scare
21.7
STEREOTACTIC/MRI
Opacity
32.8
ULTRASOUND/MRI
Spiculate mass
12.0
ULTRASOUND/MRI
The routine use of radioactive tracer techniques to guide identification and excision of breast lesions such as nonpalpable nodules, radial scare or microcalcifications, both as a diagnostic and therapeutic tool, as well as the biopsy of sentinel lymph node, both favor the increase of radioguided breast surgery [9].
Radioguided surgery (RS) is currently the safer and better performing method of detection and excision of nonpalpable lesions and sentinel lymph node for the breast surgeon. This method is based on the injection of an isotope, with known biological behavior, releasing radiation that is detected by a probe and involves intense collaboration between surgeon, nuclear physician and radiologist.
RS contemplates the radio-occult lesion localization (ROLL), the identification and the biopsy of sentinel lymph node and the sentinel node occult lesion localization (SNOLL) that combines both techniques at the same time [10].
Currently, in agreement with nuclear physicians, RS is also defined as innovative „Guided intraOperative Scintigraphic Tumour Targeting (GOSTT)” [11].
8.2 ROLL Technique
8.2.1 Introduction
The ROLL technique was developed at the European Oncologic Institute in Milan in the first half of the 1990s [12] and then refined according to different modalities, supplanting the wire guide localization (WGL) in terms of precision, accuracy and efficacy [4].
This method derives its advantage from the accuracy in locating nonpalpable lesions through an intralesional injection of a small amount of a radioactive tracer, which is injected more frequently during US, but also by stereotactic guide, depending on whether nodes are sonographically visible, microcalcifications present or clips (metal gell-mark, hydromark) left in place after mammotome study or core biopsy (Fig. 8.1). The radioactive tracer is subsequently identified intraoperatively by a gamma probe [13].
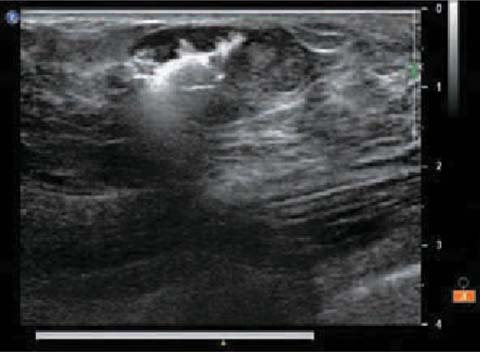

Fig. 8.1
ROLL: injection of the radioactive tracer in the breast node by ultrasound guide
The dose involves the injection of 99mTc-MAA 4 MBq in 0.1 cc followed by 0.1 cc of air and by 1 cc of iodium contrast if the injection was done under stereotactic guide. The size of albumin aggregates are 100–150 nm in dimension in order to prevent the migration of radioactive tracer guaranteeing its permanence at the injection site. The use of a proper probe for the detection of gamma radiations in the form of digital (strokes per second-sps) or acoustic signal allows the intraoperative localization of the inoculated lesion and its precise surgical radioguided resection [14].
8.2.2 Indications
ROLL is indicated in the presence of nonpalpable lesions detected in US, mammographically or after magnetic resonance of the breast.
Nonpalpable breast lesions can be detected as sonographically visible nodes or as clusters of microcalcifications, parenchymal alteration such as radial scar or a clip left after previous minimally invasive diagnostic procedures for histological typing, such as tru-cut and core biopsy by US or stereotactic guide with mammotome or magnetic resonance (MR).
8.2.3 Technical Execution
ROLL is a composite multidisciplinary procedure based on multiple connected steps and is carefully performed in order to obtain the highest effectiveness. The execution steps are summarized as follows:
Intralesional injection of the radioactive tracer under ultrasound guide and direct verification of the centering
In case of stereotactic centering, a minimal amount of water-soluble radioopaque contrast with mammographic verification of occurred centering
Capturing of the scintigraphic image
Radioguided surgical excision
Intraoperative radiography of the removed surgical specimen (Fig. 8.2)
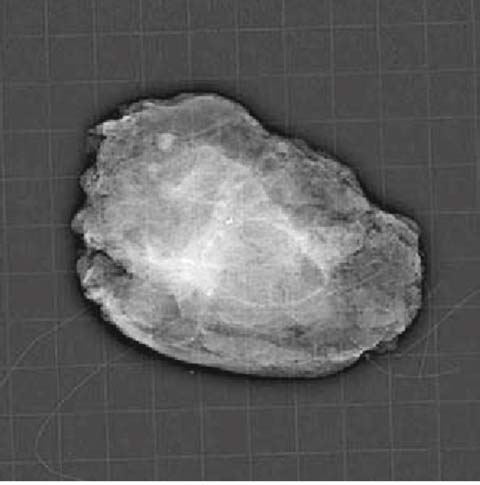
Fig. 8.2
ROLL: x-ray of the surgical specimen with clip after mammotome
Histological exam.
Therefore, there are specialized skills involved in the implementation and in the performance of the procedure: radiologist, nuclear physician, surgeon and pathologist. Multidisciplinarity, combined with a proper organization that respects given procedures, are the key requirements for a proper execution of ROLL [14].
Injection of the radioactive tracer is performed by the nuclear physician in collaboration with the radiologist. The radioactive tracer consists of about 0.05 mg human albumin macroaggregates with diameter ranging from 10 to 150 microns linked with 4 MBq of 99m-Technetium (99mTc) diluted in 0.1–0.2 cc of saline solution. The injection is performed inside the lesion or in of the area corresponding to the microcalcifications or clip, under US or stereotactic guide, either in the morning or afternoon, or even only a few hours before the operation according to organizational needs. In case of US centering, verification of the proper centering is directly and immediately revealed by the structural modifications of the lesion consequent to the injection of 0.1–0.2 cc of air following the radioactive tracer. For lesions stereotactically centered, 0.1–0.2 ml of water-soluble radio-opaque solution is also injected in order to be able to subsequently verify the proper centering with mammography [13].
Human albumin aggregates that are contained in the tracer used for ROLL are of a dimension that prevent its migration through the lymphatic vessels and keep it permanently trapped at the site of injection for at least 2436 hours.
Breast scintigraphy in anteroposterior and lateral positions performed after injection is mandatory to verify the proper and punctiform centering of the lesion with reference, for example, to the nipple, the breast fold or to the axilla. Also any possible spills with skin contamination must be highlighted, any possible intraductal or intravascular spread with the presence of other or multiple areas of radioactivity that would make difficult or impossible the identification and then the excision of the centered lesion [13].
8.2.4 Surgery
The equipment used in the localization of the radioactive tracer in the RS involves the use of a radioactivity detector in the form of a probe made by a metallic cylinder containing inside a crystal scintillator or a detector solidon (Ca, Zn, tellurium), capable of detecting gamma radiations released by 99m-Tc and transforming them into an electrical signal. The probe is connected by wire or a bluetooth wire fire system to an external processor converting the recorded radioactivity either into a digital signal (sps) readable on a display or into an acoustic signal with intensity and frequency proportional to the radioactivity captured over the investigated area [12] (Fig. 8.3).


Fig. 8.3
Device for detecting acoustic and digital signal
In the operative room, initially the probe can be used by slowly passing over the surface of the breast in a perpendicular fashion to identify the orthogonal projection on the skin of the lesion itself and to highlight it with a dermographic pencil. In this way, the surgeon can choose the more appropriate incisional site and type, according to the position of the lesion.
Radial incisions are preferred in the case of intraductal calcifications, highly suspicious lesions, and localized lesions in the lower quadrants. While arcuate incisions are mostly preferred for lesions localized in the upper quadrants. Incisions around the nipple, with an excellent esthetic result, are preferred, if possible, for all benign lesions.
Once the incision has been carried out, the probe, inserted in a sterile sheath, is from time to time moved and positioned on the surgical field in order to verify that the higher intensity of the signal is always at the center of the part being excised.
The surgeon, reading the LED-display or listening to the intensity of the acoustic signal, is able to precisely identify the area with the highest intensity of radioactive signal and then guide the surgical resection around it.
Once the specimen is removed, the probe can immediately verify whether the capitation is highest in the center. Reintroducing the probe into the surgical field of the performed resection the surgeon will be able to verify the absence of residual signal as proof of the complete removal of the previously centered lesion (Fig. 8.4).
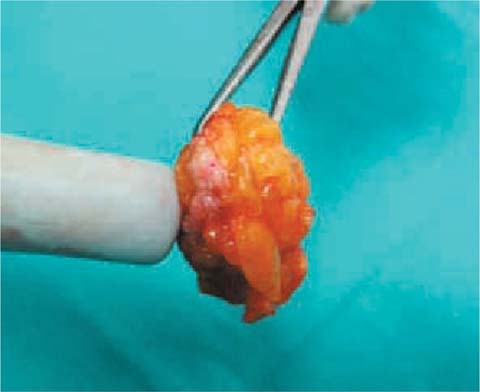

Fig. 8.4
ROLL: uptake occurs on the node removed
The specimen is then x-rayed preferably on a grid or either oriented defining the surgical margins with clips or stitches to make the pathologist’s task easier.
The adequate centering and excision procedure in RS of nonpalpable lesions allows a greater than 98% retrieval rate, making ROLL the ideal surgical procedure for nonpalpable breast lesions [12].
8.3 Sentinel Lymph Node Biopsy (SLNB)
8.3.1 Introduction
In the early 1990s, Krag, by using an intraparenchymal injection of technetium- 99m sulfur colloid, was the first to investigate radioguided sentinel lymph node technique in breast surgery [15]. The procedure has since been refined at the European Oncologic Institute in Milan [16, 17].
This method of locating the sentinel lymph node using a radioactive tracer has been rapidly adopted by breast surgeons, thus reducing morbidity, operative time and hospital stay [16, 17]. Several randomized trials in the late 1990s have established the efficacy of information resulting from the SLNB as important data among prognostic factors of BC [17]. This is based on the proven assumption that the metastatic involvement of the axillary nodes proceeds in a progressive fashion from the first to the third level of Berg and that the skip of a level can only occur only in exceptional cases [18]. The sentinel lymph node is, as a matter of fact, the first lymph node to which the primary tumor relays lymphatic drainage.
Radioguided SLNB is currently considered the method of choice in staging BC in order to avoid useless axillary dissection, which is potentially harmful due to the related side effects [15, 17].
8.3.2 Technical Execution
The SLNB involves the use of a radioactive tracer that is injected in the breast the day before surgery. It is made of colloidal particles of human albumin, approximately 80 nm (Nanocoll ©) in diameter, labeled with technetium-99m. The injection consists of 40–50 MBq of 99m-Tc-nanocol in 0.1 cc of solution. The injection can be performed perilesionally or at the subdermal level on the skin with an orthogonal projection of the nodes or alternatively at the subdermal in the periareolar area [16, 17].
The periareolar subdermal injection site guaranteed a better propagation through the lymphatic drainage, the peritumoral route may have a slower propagation and delayed highlighting of the sentinel lymph node, but it has the advantage of detecting any other extra-axillary drainage pathways such as those of the internal mammary chain; while the one on the skin projection may interfere with possible association of albumin macroaggregate used in the localization of the lesion itself if a radioguided surgical procedure is done at the same time [26].
After administration of the radioactive tracer, it is preferable to perform a quick and gentle massage over the injection site to facilitate migration of the tracer itself.
couple of hours after the administration, a lymphoscintigraphy must be performed to obtain planar images through a gamma camera equipped with high resolution collimator. Images are obtained in anterior and oblique-anterior projection. After identifying the sentinel lymph node location, its position is marked on the orthogonal skin projection with a dermographic pencil. The ideal interval between the administration of the radiocolloid and the surgical biopsy of sentinel lymph node, ranges between 3 and 20 hours [26].
In specific cases (poor display of sentinel lymph node or localization in anomalous areas), it can be useful to obtain images with a SPECT-CT technique [27].
Lymphoscintigraphy can also highlight multiple sentinel lymph nodes (even up to four), all enhanced and highlighted during the surgical biopsy. If the lymphoscintigraphy does not clearly show the sentinel lymph node, one more injection can be performed preferentially at a subdermal periareolar site. Further failure in displaying a sentinel lymph node can suggest a massive lymphangitic metastatic invasion so it will be necessary to opt for a complete axillary dissection.
Hence, some parameters need to be considered in the administration of radiocolloid: the site of injection, the volume of the radioactive tracer and the interval between the injection and the surgical procedure.
8.3.3 Surgical Technique
With the support of lymphoscintigraphy, radioguided SLNB is a rather simple technique. It can be performed either under local anesthesia or general anesthesia. SLNB can be performed before, after or at the same time as tumor surgical treatment. The organizational aspects and modalities of pathologic evaluation of the sentinel lymph node obviously affect the choice [17] (Fig. 8.5).
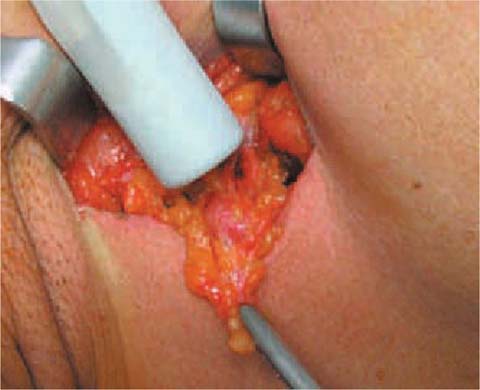

Fig. 8.5
Sentinel node: intraoperative biopsy
When pathological evaluation of the sentinel lymph node is performed as a definitive examination, it may be preferable to perform SLNB under local anesthesia in outpatient surgery before the operation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






