Psoriasis – Biologics
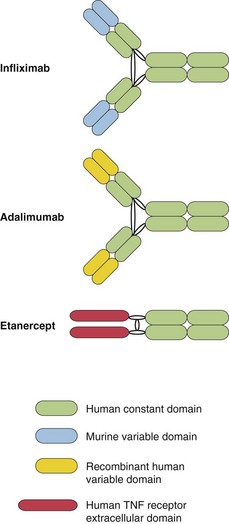
The biological response modifiers are breakthrough treatments for moderate-to-severe psoriasis and for other diseases such as rheumatoid arthritis and Crohn’s disease. Biologics are based on recombinant cytokines, fusion proteins or monoclonal antibodies (mouse or human) that bind to tumour necrosis factor-α (TNF-α), cytokine receptors or block T cell receptors to have their effect (Fig. 1).
Scientific background
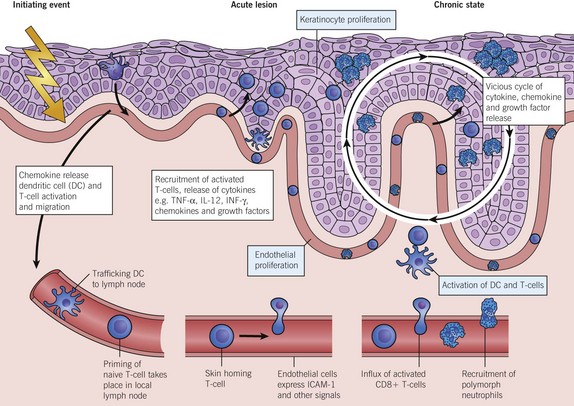
Research has confirmed psoriasis to be an immune-mediated inflammatory disease and has identified associated genes that are involved in immune regulation. These discoveries allow the pharmaceutical industry to design drugs that should work in psoriasis and in other diseases where the pathomechanisms are well understood (Fig. 2). The ‘biologics’ work by having a blocking effect on TNF-α, other cytokines (e.g. interleukin (IL)-12 and -23), dendritic cells and T lymphocytes involved in causing the disease. TNF-α is derived from Th1 cells and mediates inflammation by initiating a cascade of cytokines. IL-12 (derived from dendritic cells) and IL-23 are central to defining T helper cell types and T cell differentiation.
Types of biologic available
The most commonly used biologics for psoriasis are the anti-TNF-α agents infliximab, etanercept and adalimumab, and the anti-IL-12/IL-23 drug ustekinumab. The biologics are very effective drugs, producing better responses than agents such as methotrexate. They are given by intravenous infusion or subcutaneous injection, at intervals that vary from twice weekly to once every 3 months. The most commonly used biologics will be discussed individually.