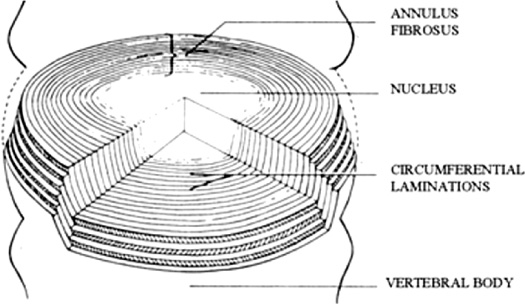
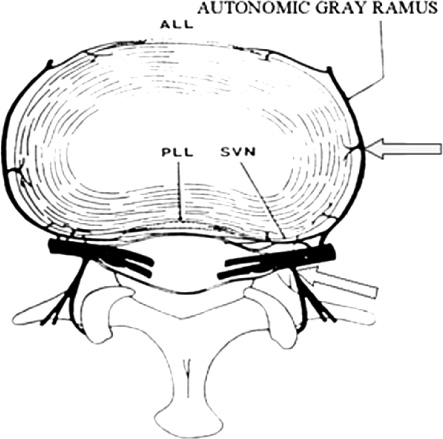
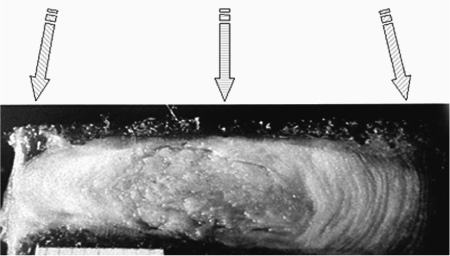
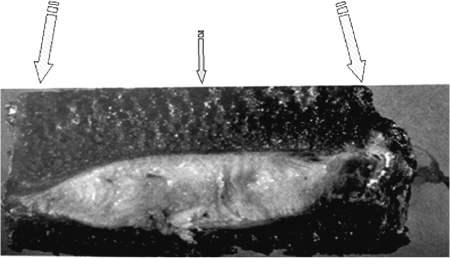
13 The normally hydrated nucleus provides annular fiber stretch, which then stabilizes the intervertebral segment. The normal pressure inside the nucleus, resulting from body weight and muscular contraction, is several times that of arterial pressure so the nucleus and internal annulus have no intrinsic circulation. The limited metabolism of these tissues is therefore essentially anaerobic. The limited nerve supply of the annulus is found only in its outer layers where the lower pressure permits neurovascular structures to exist. Studies have shown that significant changes in fluid transmission through the end plate are the likely initiator of the degenerative cascade. This progressive deterioration and dehydration of the nucleus with associated buckling of the circular laminated annulus and narrowing of the disk often lead to mechanical disruption of the annulus structure with both mechanical and chemical irritation of its outermost belt of free nerve endings. These effects often result in both diskogenic pain and segmental instability. At present, the term partial disk replacement (PDR) is used in reference to prosthetic nucleus devices, although in the future, prosthetic annuli or end plates might utilize a similar nomenclature. Prosthetic nuclei function by obliterating the degenerated toxigenic nucleus while replicating its lifting force, restoring annular tension. This uplifting force generally reduces torsional instability with its traction on the free nerve endings, restabilizes the motion segment, and expectantly halts further degenerative changes. This chapter reviews the rationale of the prosthetic disk nucleus PDR device design based on pathophysiological and mechanical parameters. Specific details of the Raymedica PDN prosthetic disk nucleus are given in Chapter 14 and elsewhere.1,2 The intradiskal nucleus tissue, well hydrated from birth until middle adult age, is principally composed of a powerful hygroscopic material, quite unlike any other body tissues. It is a complex of saccharides (polysaccharide sugars) and amino acids. This glycosaminoglycan has a core of hyaluronic acid, the most powerful hygroscopic tissue in nature, plus a small content of short collagen fibers. These tissues are synthesized in situ under optimal conditions. From fetal life onward the nucleus is normally completely surrounded and isolated from other body fluids by a multilayered, complex laminated type I collagen belt, the annulus (Fig. 13–1). In that the internal pressure within the nucleus is far higher than arterial, the nucleus tissue is essentially anaerobic and markedly hypoxic (Fig. 13–2). Vertical loading, osmotic diffusion, and normal motions of the disk aid in transferring metabolites in and out of a small microvascular bed outside the end plates adjacent to, but independent of, the quasi-venous circulation of the vertebral spongiosa. This exchange of fluids across the end plates is slow (having a 4- to 8-hour cycle) and produces an approximate 15 to 20% daily change in both gel hydration and associated volume, or a rise and fall of ~1 to 2 mm in disk height per lumbar segment, depending on spine loading. The transmission of nutrients effectively filters out large circulating fractions from the blood, resulting in an isolation of the nucleus tissue. The incoming filtered nutrients are partially catabolized into by-products that are anaerobic, neurotoxic, and potentially pain producing. But, when these products pass out through the end plates they become diluted as they enter the microcirculation mentioned earlier. The nucleus can thus be thought of as the engine of the disk where the end plate microcirculation provides its fuel. Alterations in the end plate transmission of nutrients and catabolic by-products are thus the most likely cause of nucleus starvation, loss of water-binding ability with associated degenerative dehydration, hyperaccumulation of the toxins, and collapse of the disk. As acid metabolites accumulate and the pH falls the synthesis and regeneration of nucleus and inner annular tissues ceases, further accelerating diskal degeneration. Figure 13–1 A cross-sectional diagram of the human disk space illustrating the circumferential laminations of the annulus fibrosus (as many as 24 at the lumbosacral level) and the contained hydrogel nucleus tissue (glycosaminoglycan). This hygroscopic tissue absorbs water and swells, exerting substantial upward and centrifugal pressure to maintain annulus height and stabilize the segment. The nucleus is normally at very high pressure (up to 6 atmospheres, ~5000 mm Hg), prohibiting any direct circulation or nerve supply. The internal tissues are therefore anaerobic and accumulate potentially toxic substances that are normally diluted through the end plates to pass into extrinsic circulation. Annular tears may release these substances to reach the outer annulus innervation producing severe diskogenic pain. Figure 13–2 Diagrammatic cross-section of a disk showing the nerve and vascular supply encircling the outer annulus. At intervals the neurovascular bundles turn and enter the outer annulus (side arrow) there to spread in the outer six circumferential layers. The location of the sensory ganglia with ventral and dorsal branches is indicated by the lower arrow. ALL, anterior longitudinal ligament; PLL, posterior longitudinal ligament; SVN, sino-vertebral nerve. With failure of transference, the by-products of normal intradiskal anaerobic metabolism can markedly increase. These include lactic acid, phospholipase A2, stromalysins, metalloproteinases, and other potentially neurotoxic substances. Should these escape via annular tears or defects and reach the free nerve endings of the outer layers of the annulus, or reach nearby nerve ganglia, severe, recurrent back and leg pain and potential spinal nerve damage can result.2 Further, degenerative delamination of layers in axial rotation can produce traction on the free nerve endings that cross the layers resulting in stretch-induced pain. As already indicated, diskogenic pain can therefore result from chemical or physical (torsional) stimulation of the polymodal pain-transmitting, free nerve endings in the outer annular belt. Nonetheless, the vast majority of degenerated disks, for a variety of interesting and in part unknown reasons, are essentially painless. The annular collapse causes a tearing of its fibers from the vertebral outer ring, resulting in torsional instability and “traction” spur formation along with the delamination of annular belts. The classical degenerative cascade of disk dehydration, collapse, loss of flexibility, and delaminated buckling as discussed earlier, as far as is now known, cannot be repaired or reversed3 (Figs. 13–3 and 13–4). Medication, exercise, spinal column traction, and all other conservative, nonsurgical means that may be generally beneficial during early degeneration do not appear to be capable of halting or reversing the progressively advancing degenerative cascade. Current standard surgical treatments of this disorder are focused on obliteration of the abnormal, frequently painful disk structures by fusion or total diskal replacement. Prosthetic disk nucleus devices, with only one of the few existing designs now in broad commercial use and others in various stages of development, were conceived as a more physiological and anatomical solution to disk degeneration. Figure 13–3 Photograph of cross-section of normal human intervertebral disk illustrating the distribution of normal, downward forces (arrows) evenly across the disk space, end plate, and epiphyseal ring margins. Nucleus hydrogel glycosaminoglycan expansion lifts the space, tightens annular fibers, and maintains flexible stability. Figure 13–4 Photograph of cross-section of degenerated human intervertebral disk illustrating the loss of supporting counterforce across the end plate due to loss of water-binding nucleus substance. Early degeneration loosens the annulus and promotes instability, but with continuing marked degeneration the space ultimately becomes stable and often rigid.3
Prosthetic Disk Nucleus Partial Disk
Replacement: Pathobiological
and Biomechanical Rationale for
Design and Function
 The Pathophysiology of the Degenerating Disk
The Pathophysiology of the Degenerating Disk
 Biomechanical Considerations for Partial Disk Replacements
Biomechanical Considerations for Partial Disk Replacements
The Pathophysiology of the Degenerating Disk
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Summary
Summary