, Paul N. Chugay1 and Melvin A. Shiffman2
(1)
Long Beach, CA, USA
(2)
Tustin, CA, USA
Abstract
All cosmetic surgery, including body implant surgery, can be performed under local anesthesia alone. More often than not, general inhalation anesthesia (GA) or propofol-opioid (i.e., alfentanil or remifentanil) total intravenous anesthesia (TIVA) is used for greater control of patient movement. Brain-monitored propofol permits the differentiation between the need for more local analgesia (spinal cord-generated movement) and the need for more propofol (brain-generated movement). Patients are universally happy with BIS/EMG PK MAC anesthesia.
Poetry in Motion
All cosmetic surgery, including body implant surgery, can be performed under local anesthesia alone. When awake patients have pain during the case, they may move, but they also communicate verbally of their inadequate analgesia, i.e., “Ouch!” (Fig. 2.1).


Fig. 2.1
Surgery without pain: an achievable PK goal
More often than not, general inhalation anesthesia (GA) or propofol-opioid (i.e., alfentanil or remifentanil) total intravenous anesthesia (TIVA) is used for greater control of patient movement. Greater patient movement control obscures vital information about inadequate local analgesia to the (postoperative) detriment of the patient. No postoperative patient benefit could be determined when preemptive local analgesia was injected after induction of GA and prior to incision as seen in a meta-analysis of 80 studies [1].
Figure 2.2 clearly illustrates brain function is not necessary to produce movement. Movement in a sedated patient may also occur with or without brain involvement. Without the ability to discern brain-generated movement from that generated from the spinal cord, one remains stuck in the twentieth century mode of anesthesia wherein all patient movement had to be treated as if it might be a sign of patient awareness or recall.


Fig. 2.2
Headless chicken generating movement
Patient movement under sedation is almost always the patient’s request for more local analgesia (Fig. 2.3). Brain-monitored propofol permits the differentiation between the need for more local analgesia (spinal cord-generated movement) and the need for more propofol (brain-generated movement). The ability to differentiate, and subsequently more appropriately treat, the two distinctly different types of patient movement results in less inappropriate types (and amounts) of adjuvant drugs being given to sedated patients.


Fig. 2.3
Adequate local analgesia is a critical element of PK anesthesia
Some surgeons direct their own diazepam (or midazolam)-ketamine anesthesia [2] with an impressive safety record. However, benzodiazepine sedation has no reliable, reproducible clinical signs for adequacy of brain protection from negative ketamine side effects. Currently available cerebral cortical monitors do not reliably measure benzodiazepine effect.
Direct measurement of anesthetic effect on the cerebral cortex has only been available since the 1996 FDA approval of the Bispectral Index® (BIS) (Aspect Medical Systems, Inc.) monitor. While cerebral cortical monitoring does not replace vital signs, like heart rate and blood pressure, vital signs only reflect brain stem activity (Fig. 2.4).
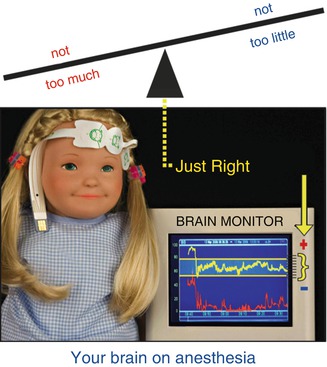

Fig. 2.4
BIS/EMG monitored PK MAC (aka “Goldilocks” anesthesia)
Brain stem activity (i.e., vital signs) simply cannot reliably guide the cerebral cortical effect, as was standard anesthetic practice prior to 1996. The net result of this void of cortical effect information was routine over-medication to prevent under-medication (anesthesia awareness). Complex activities like processing hearing, feeling, and recall occur in the cerebral cortex. Clearly, then direct cerebral cortical monitoring should be part of the twenty-first century anesthetic practice.
Over the past 21 years, propofol-ketamine (PK) monitored anesthesia care (MAC) has appeared as an alternative to both GA and propofol-opioid TIVA. From March 26, 1992 through December 25, 1997, PK anesthesia was more art than science. With the addition of BIS/EMG monitoring on December 26, 1997, numerical reproducibility was achieved [3].
Propofol-Ketamine TIVA [4] or “Ketofol”
There is no precise definition of what ketofol is. Generally ketofol refers to the 50:50 mixture of ketamine and propofol, 0.5 mg/kg of each (Fig. 2.5). However, a broader definition considered that ketofol is the combination of ketamine and propofol, regardless of the ratio to each other (the initial dose of each can be scaled up to 3 mg/kg). When they are used in infusion, the dose is 100 μg/kg/min.


Fig. 2.5
Vodka martinis illustrating the difference between PK MAC and PK TIVA (aka ketofol)
The principle objection to ketofol is the inability to ascertain the amount of hypnosis (propofol effect) and the degree of NMDA block (ketamine effect) with induction and prior to the initial local anesthetic injection. The secondary objection is the potential for exceeding 200 mg ketamine during the case, potentially prolonging emergence. Conversely, PK MAC clearly defines hypnosis (BIS <75, baseline EMG) prior to dissociation (immobility with injection).
Anesthesia considerations for body implant cosmetic surgery revolve around the three parties’ concerns – the patient, surgeon, and anesthesiologist. The key consideration is that the patient is the first priority! The patient wants (1) not to hear, feel, or remember their surgery, a cerebral cortical effect, and (2) to awaken promptly without pain, prolonged emergence, or postoperative nausea and vomiting (PONV) (Fig. 2.6), a function of anesthetic technique.


Fig. 2.6
Emesis is our nemesis
The surgeon wants a motionless patient during the surgery and the fewest possible postoperative concerns. Numb patients rarely move under sedation. Without a brain monitor to assure adequate propofol sedation levels (i.e., BIS <75, EMG baseline vide infra), it is nearly impossible to encourage the surgeon to re-inject a vasoconstricted field.
The anesthesiologist wants reproducibility along with control of the patient’s airway and movement during surgery. However, this is confounded by variations in patients’ cerebral tolerance to medication effect in addition to their varying ability to metabolize and eliminate the anesthetic agents.
Friedberg’s Triad answers the patients’ desires, the surgeon’s needs, and the anesthesiologist’s quandary over what drug or intervention is most appropriate when facing patient movement under sedation.
1.
Measure the brain
2.
Preempt the pain
3.
Emetic drugs abstain
“Measure the brain” means incrementally titrating propofol to BIS <75 with baseline electromyogram (EMG) (Fig. 2.7). Brain measurement provides numerically reproducible propofol levels to protect the brain from negative ketamine side effects.
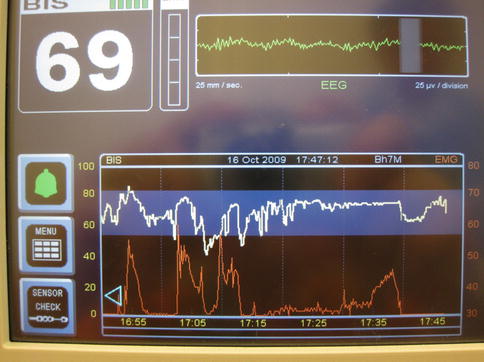

Fig. 2.7
EMG is the lower (red) trace
“Preempt the pain” means using a 50 mg dissociative dose of ketamine, independent of adult body weight, to completely saturate midbrain NMDA receptors 3 min prior to skin stimulation (i.e., injection of local analgesia).
“Emetic drugs abstain” simply means scrupulous avoidance of opioids (narcotics) like morphine, meperidine, fentanyl, alfentanil, or remifentanil as well as inhalational agents like forane, sevoflurane, or desflurane (Fig. 2.8). Assurance of adequate local analgesia obviates the need for these troublesome agents. Abstaining from emetic agents also eliminates the need for antiemetic drugs.


Fig. 2.8
“As long as emetogenic agents are part of the anesthetic regimen, the use of anti-emetics is of limited utility” – Christian Apfel MD, PhD
Propofol’s advantages over inhalational agents include the following:
1.
Not a malignant hyperthermia (MH) trigger.
2.
Not needing to stock dantrolene, an MH antidote.
3.
Antiemetic qualities.
4.
Antioxidant qualities: halogenated inhalational agents like forane, desflurane, or sevoflurane are oxidizing agents.
5.
Rapid, pleasant emergence likely due to rapid metabolism.
6.
Preserved REM sleep patterns.
Unlike benzodiazepines, propofol clinical signs (i.e., loss of lid reflex and loss of verbal response) are reliable and clinically reproducible, and cerebral cortical effect can be measured and, therefore, is numerically reproducible.
Bispectral Index (BIS) Monitor
With the 1983 introduction of pulse oximetry (SpO2) monitoring, anesthetic mortality declined from 1 in 10,000 in the 1950s to about 1 in 250,000 patients. Additional vital signs of blood pressure, EKG and EtCO2, while important, still only provide a reflection of brain stem activity. However, the part of the brain that processes hearing, feeling, and memory is the cerebral cortex.
For anesthesiologists practicing prior to 1996, there was no direct measure of patient cerebral anesthetic response. To compensate for this lack of information about cortical effect, the anesthesiologist was obliged to over-medicate for fear of not giving enough anesthetic. In 1996, the Food and Drug Administration (FDA) approved Aspect Medical Systems’ Bispectral Index (BIS) monitor to directly measure the patients’ cerebral drug response.
While the BIS technology has been validated in over 3,500 published scientific studies and found in over 75 % of US hospitals, BIS utilization remains at only about 25 %. There are several reasons for the underutilization of BIS monitoring. First, the BIS value is a unit-less, derived value that is 15–30 s behind real time (vital signs, like heart rate and blood pressure, are measured in real time). Titrating anesthetics with only the BIS value is akin to trying to drive an automobile with only the rearview mirror information. The factory default setting displays only the BIS value and tracing. A tool that does not provide useful, real-time information is not often used. BIS values delayed from real time are not especially helpful to titrate anesthetics.
The optimal use of BIS is by trending the frontalis muscle electromyogram (EMG) as the secondary trace [5] and responding to EMG spikes as if they were heart rate or blood pressure changes. EMG is to the frontalis muscle what the EKG is to the myocardial muscle, i.e., a real-time, physiologic parameter (Fig. 2.9). While some allege the use of ketamine invalidates the ability to titrate propofol with BIS, there is evidence to the contrary [6], along with 15 years of reproducible clinical practice.
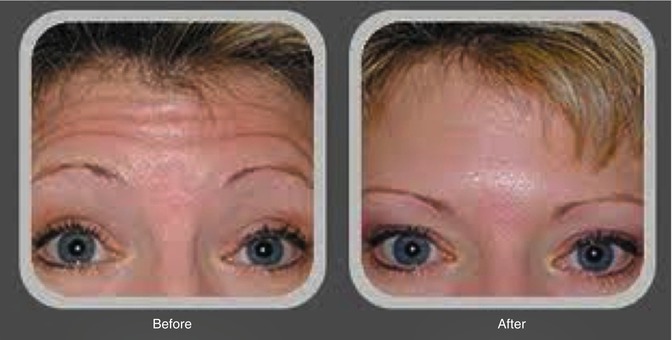

Fig. 2.9
Botox does not eliminate EMG spikes
Premedication
Between March 1992 and June 1997, the addition of midazolam premedication was undertaken in the hope of reducing the cost of the average 3–20 ml bottles of propofol (Diprivan® Zeneca). Three groups of patients were informally studied – 0, 2, and 4 mg midazolam premedication, with the 4 mg group selected for cases of 4+ h. Review of the comparative propofol rates revealed no cost-effective reduction with either 2 or 4 mg midazolam premedication [7].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








