Major criteria (Required for diagnosis)
Any pruritic skin condition in the past 12 months
Minor criteria (Require 3 of the following)
Age of onset younger than 2 yearsa
History of flexural skin involvement
History of generalized dry skin
Personal history of other atopic diseaseb
Clinical flexural dermatitis documented by photographic protocol
Although the pathogenesis of AD remains to be fully deciphered, this multi-factorial disease is likely a product of both genetic and environmental factors. Data from human and animal studies indicate that a Th2 cell immune response is dominant during the acute phase of AD, and that a Th1 cell response is prevalent during the chronic phase [5–7]. In addition, infiltration of inflammatory cells, defective epidermis barrier functions, and high levels of inflammatory mediators are typically associated with AD [5, 6]. The current approach towards the management of AD has mainly been to suppress the inflammatory nature of the disease. Although this approach helps to control the inflammation and the symptoms of AD, it does not cure the chronic problem.
Recent studies of factors involved in inflammatory disease have focused on angiogenesis and lymphangiogenesis. The process of neovascularization has been found to be important in other inflammatory disorders like rheumatoid arthritis, psoriasis and some autoimmune diseases [8]. Since blood vessels are the essential pathways for transporting immune cells (T cells, eosinophils, dendritic cells and macrophages) to the site of inflammation, examining the role of vasculature in the inflammatory skin diseases seems to be a logical approach. Pro-inflammatory mediators such as selectins, cell adhesion molecules (CAM), integrins, cytokines, chemokines, as well as vascular endothelial growth factors (VEGF), have been associated with the process of new capillary formation. As angiogenesis and lymphangiogenesis are the development of microvasculature in response to inflammatory agents, these two phenomena could in theory play some roles in the pathogenesis of AD – as AD is a prototypic chronic inflammatory disease [9].
Currently, there is a scarcity of research studies that demonstrate angiogenesis or lymphangiogenesis in human AD. In the following sections, our review on human AD will be primarily on the role of vasculature in AD development. We will then review the strong evidence of angiogenesis and lymphangiogenesis in an animal model of AD. By understanding the potential roles of vasculature and neovascularization in AD, we may be able to open a novel path to therapeutic intervention.
Evidence of Angiogenesis and Lymphangiogenesis in Human Atopic Dermatitis
Selectins
The early steps of the inflammatory process involves leukocyte extravasation. Evidence suggests that upon antigenic stimulation, resident macrophages in the affected tissue release cytokines and chemokines such as IL-1 and TNFα and CCL-5 which attract circulating leukocytes to the site of injury. TNF alpha and IFN gamma then induce the endothelial cells at the activated site to express adhesion molecules called selectins. These molecules allow for the tethering and adhesion of leukocytes to the vascular endothelium [10]. Upon stimulation, L-selectin, found on all circulating leukocytes, and E- and P-selectins, expressed on the activated endothelial cells, will bind to each other’s complementary carbohydrate ligands with marginal affinity. This will facilitate the process of rolling and tethering of the leukocytes along the inner surface of the blood vessel walls. Research has indeed shown that E-selectin levels are elevated in lesional skin of adults and children with AD [11]. There is also a positive correlation between the number of mononuclear cells and T cells with the degree of E-selectin expression in lesioned skin of human AD [11]. In the CD4 T cells of AD patients, an increase in L-selectin level was also detected [12]. Because of this data, selectin levels have been suggested as a biomarker for AD disease activity as well as treatment response [13].
Immunoglobulin Superfamily Cell Adhesion Molecules
Another subset of the cellular adhesion molecules are a large group of cell surface proteins involved in the recognition and binding of cells known as the immunoglobulin superfamily cell adhesion molecules (IgSF CAMs). These proteins have domains that are similar in structure to antibodies and can activate leukocytes, thereby enhancing their adhesion to other leukocytes and endothelial cells. The IgSF on endothelial cells, ICAM-1, 2, 3 and VCAM-1 are expressed at different phases of the inflammatory process and bind to their respective β2 integrin ligands to initiate adhesion [12]. While VCAM-1 and ICAM-1 are minimally expressed in the blood vessels of non-atopic individuals, they are strongly expressed in the non-lesional skin and expressed with greater intensity in lesional skin of AD patients, suggesting their involvement in the inflammatory process [14].
New cell adhesion molecules have emerged in the study of AD pathophysiology. One molecule of interest is the vascular adhesion protein-1 (VAP-1), which was discovered in the late 1980s to be involved in the inflammation of arthritis as well as in lymphocyte migration in inflamed skin and the gut [15]. It also participates in the adhesion cascade of multiple tissues by allowing the lymphocyte-endothelial cell interaction to occur. One study showed that VAP-1 was overexpressed in both the lesional and non-lesional skin biopsies of AD patients. The fact that the serum levels of VAP-1 decreased after AD therapy also suggests a role of VAP-1 in the AD disease process [15].
Integrins
Mechanistically, certain chemokines released by macrophages could cause cellular transmembrane receptor molecules integrins to switch from a low-affinity state to a high-affinity state. Once activated, integrins will bind tightly to complementary receptors expressed on vascular endothelial cells with high affinity allowing for a firm attachment of the leukocytes. This action functions to prevent WBCs from being swept away by the shear forces of the ongoing blood flow. Similarly, integrins on eosinophils’ cell surface can also recognize the laminin, fibronectin, and glycosaminoglycans hyaluronic acid of the extracellular matrix allowing the eosinophils to firmly attach to the sites of inflammation [16]. Of note, the α6 integrin, found on the luminal side of blood vessels, have been studied for its role in binding migratory T cells in atopic skin. A significant up-regulation of the α6 integrin has been found on the endothelial cells and in the epidermis of lesional skin of AD as compared to control, supporting a role of this integrin in the inflammatory process of AD [17].
Transmigration
The entire process of diapedesis, the transmigration of the leukocytes out of the blood vessels, is well coordinated between leukocytes and endothelial cells. After adhesion to the endothelial cells, leukocytes rearrange their cytoskeleton so that they can flatten and spread out over the endothelium. In this morphology, leukocytes can extend their pseudopods and pass through the gaps created between neighboring endothelial cells. Platelet endothelial cell adhesion molecules (PECAM-1) proteins found on both leukocytes and endothelial cells can facilitate the movement of the leukocytes out of the endothelium [17]. Transmigration of the leukocyte continues with breaking through the basement membrane by way of either proteolytic digestion of the membrane and or mechanical forces. Once in the interstitium, leukocytes resume their travels towards the site of injury or infection via chemotactic agents. Significantly, the expression of PECAM-1 is highly increased in the lesional skin of AD patients as compared to uninvolved atopic skin [18].
Growth Factors in Angiogenesis and AD
Growth factors involved in vascular remodeling have been identified with inflammatory disorders [19]. One growth factor of interest in inflammation-mediated angiogenesis is the vascular endothelial growth factor (VEGF), which is present in four different isoforms (A, B, C and D). As the name suggests, VEGF promotes the growth, survival and migration of endothelial cells and is a potent vasodilator. The A and B isoforms are angiogenic in nature with VEGF-A being the most pro-angiogentic isoform, while the C and D isoforms are involved in lymphangiogenesis [20]. VEGF signals through three human VEGF receptors (VEGFR-1,2,3) [21] and two co-receptors, Neuropilin-1, 2 (NRP-1,2) [22]. NRP-1 is mainly expressed on arterial endothelial cells and has the binding affinity for VEGF-A [23]. NPR-2 is found on venous and lymphatic endothelial cells [24], thereby supporting lymphangiogenesis. By changing the vascular permeability, remodeling the vascular tone, and increasing blood flow and surface area to promote leukocyte-endothelial cell interactions, VEGF contributes to the angiogenic process. Its increased expression in the epidermis of AD patients has been documented [25]. The ability to increase blood vessel dilation and permeability, as well as being a chemotactic factor for monocytes, may make VEGF a key participant of inflammatory disease in general and of AD onset and development in particular [26]. In fact, researchers confirmed that the levels of the stratum corneum VEGF not only correlated with the disease severity but also with the levels of serum LDH and the amount of eosinophils in AD patients [25].
It has been suggested that single nucleotide polymorphisms (SNPs) may be associated with VEGF overexpression. Shahbazi et al. showed that at position 1154 on chromosome 6p12, the GG and GA genotypes of the VEGF gene is linked to higher VEGF production by mononuclear cells [27]. By digging deeper into gene polymorphism, other have discovered that AD patients have greater GA expression at position 1154 of the promoter region of the VEGF gene which may be accounted for the higher levels of VEGF expression.
Mediators Involved in Angiogenesis and AD
Mast cells
Increasing experimental evidence suggests that inflammatory cells contribute to the development of AD by creating angiogenic and lymphangiogenic factors. Human mast cells are a likely contributor because of their close proximity to epidermal endothelial cells and because of their abilities to release pro-angiogenic factors and histamine which can trigger increased vascular permeability, smooth muscle contractions and vasodilation. Investigators have demonstrated that human skin mast cells, which are abundant in the lesional skin of AD patients, contain and release VEGF-A, B, C and D as well as the various VEGFR, thereby serving both as a source and a target for VEGF. [28] From this observation, it was postulated that the VEGF made by the mast cells can recruit more mast cells to the site of inflammation in a paracrine fashion in order to sustain the angiogenic process in AD. [20]
Mast cells are also known to release tryptase, an enzyme that can activate the receptor proteinase-activated receptor 2 (PAR2) on the dermal endothelium resulting in the up-regulation of ICAM-1 and E-selectin. The levels of tryptase and PAR2 are observed to be elevated in the lesional skin of AD patients suggesting localized mast cell involvement in skin inflammation. [29]
The close proximity of epidermal and papillary mast cells to endothelial cells may allow mast cells to participate in cutaneous inflammation and angiogenesis. CD31-positive endothelial cells were found to grow only in the company of surrounding mast cells from the papillae up to the stratum granulosum. Even more interestingly, mast cells may exhibit a bidirectional “tunneling” effect in the epidermis of AD patients. In the “forward” direction, mast cells secrete matrix-degrading enzymes like MMPs which clears out a tunnel in the epidermis. While in the “backwards” direction, mast cells secrete pro-angiogenic factors, so that new vessels can be attracted to sprout into the tunnel behind it. [30] In fact experiments have shown an increase in MMP-2 expression along with several pro-angiogenic factors in the presence of mast cells in AD skin. [30]
Cytokines
Cytokines and endothelial cells are co-factors in inflammation. Various interleukins, such as IL-1, 4, 6, 8, 9 and 17, have been identified with special contributions to vascular regulation and AD. [12, 31] IL-1 and TNF-alpha both up-regulate ICAM-1 and E-selectin, resulting in the activation of dermal vascular endothelial cells. IL-17 further augments this effect by inducing more IL-1 secretion from the same endothelial cells. IL-17 also enhances the actions of fibroblast growth factor-beta (FGF-β) and VEGF, stimulating angiogenesis and endothelial cell migration. [32]
IL-4 plays an important role in the pathogenesis of AD. It has been shown to up-regulate AD related chemokines CCL25, CCL26, pro-inflammatory factors IL-1, IL-19 and IL-20 as well the pro-angiogenic factor VEGFA in human keratinocytes [31], thus providing a link between angiogenesis and atopic dermatitis.
Various studies have cited that AD patients have increased levels of IL-8 in the plasma and eosinophils. IL-8 is also known to mediate angiogenesis by increasing endothelial cell proliferation and chemotaxis. [20]
IL-9 was initially defined as a T-cell and mast cell growth factor associated with the Th2 immune response. It has only recently been reported to promote VEGF release from mast cells through STAT3 activation. [33] IL-9 and IL-9 receptor mRNA expressions are in fact highly elevated in the lesioned skin of AD patients as compared with controls. [33] These data are revealing of IL-9’s role in both angiogenesis and AD.
IL-6 is a well-known mediator of inflammation and is produced by endothelial cells. IL-6 in turn acts on endothelial cells to further increase their growth and activation. Up-regulation of IL-6 is not only found in human AD but can cause inflammation in various other diseases. [12]
Chemokines
Chemokines are involved in both the adhesion and trans-membrane migration segments of the leukocyte extravasation process in the endothleium. In AD, endothelial cells, keratinocytes, and fibroblasts can provide a variety of chemokines to attract leukocytes. [12] For example, chemokines such as CCL11, CCL13, CCL17, CCL18, CCL22, CCL26 and CCL27 have higher expression in the skin of AD patients as compared to normal subjects. [34] CCL-1, 11 and 26 can interact with endothelial cells to stimulate angiogenesis leading to further exacerbation. [12]
Evidence of Angiogenesis and Hymphangiogenesis in a Mouse Model of Atopic Dermatitis
Generation and Characterization of the Model
Since studies of angiogenesis and lymphangiogenesis in human AD are limited in scope and in depth, we next examine the evidence from an animal model. Developed in 2001 by Chan et al., the Keratin-14-IL-4 Transgenic (IL-4 Tg) mouse line is the only known AD experimental animal model with characteristic chronic inflammation. [35] The IL-4 Tg mouse was established by over-expression of IL-4, a critical Th2 cytokine involved in AD pathogenesis, in epidermal keratinocytes using a Keratin-14 promoter/enhancer. The IL-4 Tg mice spontaneously developed pathologic phenotypes that fulfilled key diagnostic criteria of AD seen in human patients. [36] Clinically, the Tg mice developed xerosis and pruritic inflammatory lesions in the ears starting at age of 4 months. Lesions later extend to the neck, mouth, eyes, back, torso, tail and legs (Fig. 6.1). Eye involvement caused eyelid dermatitis, blepharitis and conjunctivitis frequently result in corneal and conjunctival scarring. Microbiologically, crusted lesions and bacterial pyoderma developed at the external ears from self-excoriation are culture-positive for Staphylococccus aureus and Pseudomonas aeruginosa, with the former being a common infectious agent leading to disease exacerbation in human AD patients. Histopathologically, when compared to Non-transgenic (NT) littermates, the Tg mice skin lesions are characterized by increased spongiosis, acanthosis, and prominent epidermal and dermal infiltration of CD3+ T cells, macrophage-like mononuclear cells, degranulating mast cells, and eosinophils (Fig. 6.2). Despite epidermal over-expression of IL-4, the serum IL-4 level remains undetectable by ELISA. Immunologically, the onset and severity of skin disease correlate with the elevation and maintenance of serum levels of total IgE and IgG1. To monitor the disease progression and to correlate its progression with immunological mechanism, the Tg mice are classified into three groups based on disease progression, Tg-BO for mice before onset of any visible skin lesion, Tg-EL for mice with early/acute skin lesions of less than 1 week duration, and Tg-LL for mice with late/chronic skin lesions of equal to or greater than 3 weeks of duration.
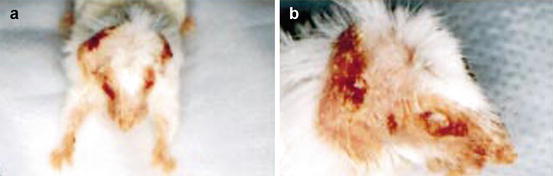
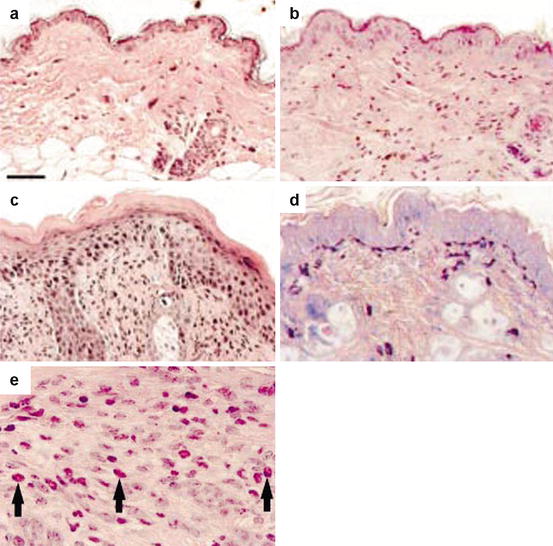

Fig. 6.1
Clinical manifestation of atopic dermatitis in affected IL-4 Tg mouse. Severe dermatitis on head and neck with areas of crusting, hair loss and bacterial pyoderma (a). External ear destruction results from self-excoriation and eyelid dermatitis leads to corneal and conjunctival scarring (b) (Reproduced with permission, Chan et al. [35])

Fig. 6.2
Histopathological findings of skin lesions. NT littermates (a) have normal epidermal thickness with rare mononuclear cells. The skin of EL IL-4 Tg mice demonstrates mild spongiosis and acanthosis, with mononuclear cell infiltrate (b). LL mice demonstrate characteristics of chronic inflammatory lesion, with prominent hyperkeratosis and dermal mononuclear infiltrate, spongiosis and focal areas of parakeratosis (c). Mast cell degranulation (d) and eosinophil infiltrates (e) are also prominently present in chronic lesions. Hematoxylin & eosin (a–c, e). Geimsa (d) (Reproduced with permission, Chan et al. [35])
Morphological Evidence
Angiogenesis occur in conjunction lymphangiogenesis in wound healing, inflammatory diseases such as peritonitis and rheumatoid arthritis. Dermal angiogenesis parallel lymphangiogenesis in psoriasis [37], a prototypic chronic inflammatory skin disease. However, limited data exist on the alteration of skin microcirculation of AD in human. Fueled by the successful introduction of the K14-IL-4 Tg mouse, much progress has been made to investigate the presence and pathogenic role of microvascular new growth in AD. First let us examine the morphological evidence.
Immunofluorescence Microscopic Evidence
Recent morphological studies revealed that both dermal angiogenesis and lymphangiogenesis are prominent features of the IL-4-Tg mouse. When compared to NT, the Tg mice have significantly higher expression of CD31 (an endothelial cell specific marker), and VEGFR-2 (VEGF receptor specific to blood vessels) per linear skin length. Furthermore, the average blood vessel diameter and skin area occupied by vessels increased as disease evolved from Tg-BO to Tg-EL and then to Tg-LL [38] (Fig. 6.3). Under confocal microscopy, Tg-EL and Tg-LL have increased dermal CD31 expression and reduced expression of claudin-5, an endothelial tight junction protein, when compared to NT and Tg-BO. This finding supports the notion that lesional skin has larger number of newly formed vessels, which characteristically have fewer tight junctions [39].
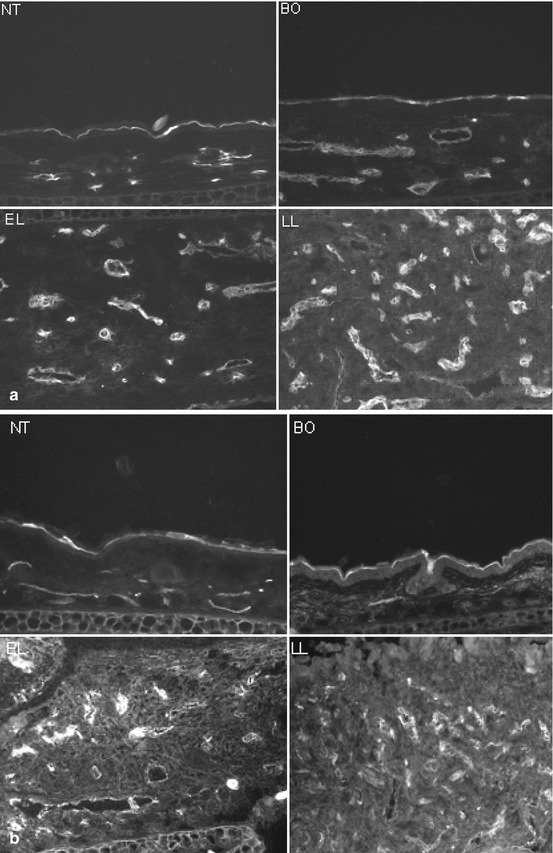

Fig. 6.3
Immunoflouresence microscopy of dermal vasculatures. CD31 (endothelial cell marker, Panel a) and VEGFR2 (VEGF receptor 2, Panel b) expressions parallel the severity of skin inflammation in the IL-4 Tg mouse. Top left, NT; top right, BO; bottom left, EL; bottom right, LL (Reproduced with permission, Chen et al. [38])
Identification of lymphatic vessel-specific markers in recent years enabled the study of inflammatory lymphangiogenesis. Dermal lymphangiogenesis parallels angiogenesis in the Tg mice. Lymphatic vessel-specific markers, such as podoplanin (PDPN), lymphatic vessel hyaluronan receptor (LYVE-1) and vascular endothelial cell growth factor receptor-3 (VEGFR-3) expressions were significantly increased in Tg mice when compared to NT mice [40] (Fig. 6.4). This phenomenon is observed even in lesion-free areas of the skin of the Tg mice. As seen in angiogenesis, the extent of lymphatic marker over-expression also correlates closely with disease severity, i.e., the severer the disease stage, the greater expression of lymphytic marker. When compared to NT littermates, the lymphatic vessels in Tg mice were greater in number, larger average and maximum diameter, and occupied more volume in the dermis. The evidence that the growth of both blood and lymphatic vessels match closely with the progression of skin lesions in Tg mice suggests a tight link between inflammatory reaction and neovascularization.
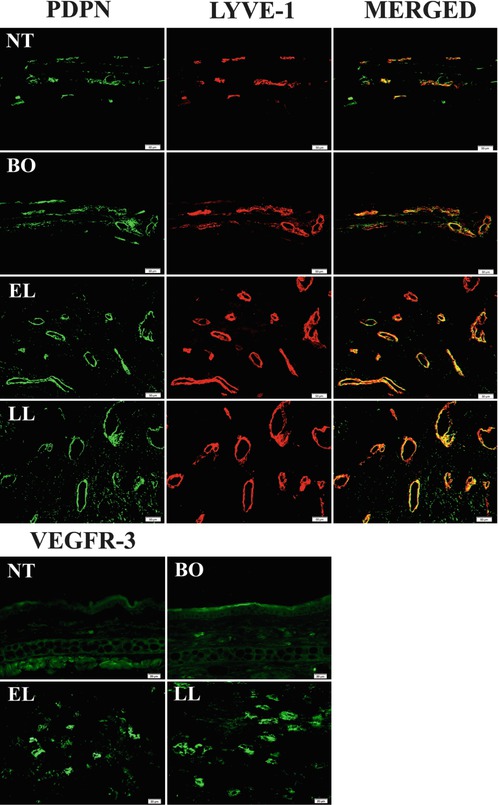

Fig. 6.4
Immunoflourescence microscopy of dermal lymphatics. Lymphatic- specific markers, PDPN, LYVE-1 and VEGFR-3 expressions are significantly increased in Tg mice when compared to NT mice. The increased expression of these three markers correlates with disease severity. Left block: PDPN (green) and LYVE-1 (red) co-localize on lymphatic endothelium (merged). Right block: VEGFR-3 expression in various disease stages. PDPN, podoplanin; LYVE-1 (lymphatic vessel hyaluronan receptor); VEGFR-3, vascular endothelial growth factor-3 (Reproduced with permission, Shi et al. [40])
Electron Microscopic Evidence
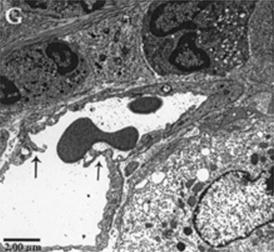
Electron microscopy (EM) studies on the skin of the Tg mice revealed important ultrastructural changes in the microvascular beds [39]. Under transitional electron microscopy (TEM), the perivascular dermal matrix of NT mice consists of fibroblasts, collage and mast cells. NT mice have dermal blood capillaries that are continuous and formed by 1-2 endothelial cells (EC) with regular and smooth surfaces (Fig. 6.5a). In contrast, Tg mice dermal EC exhibit progressive nucleoli hypertrophy, cytoplasmic vacuolation, organelle hyperplasia, and increase in cell surface irregularity, thus demonstrating an endothelial cell activation process (Fig. 6.5b–d). The extracellular matrix is also more edematous with increased erythrocyte extravasation. These ultrastructural alterations documented in the Tg mice are hallmarks of angiogenesis.
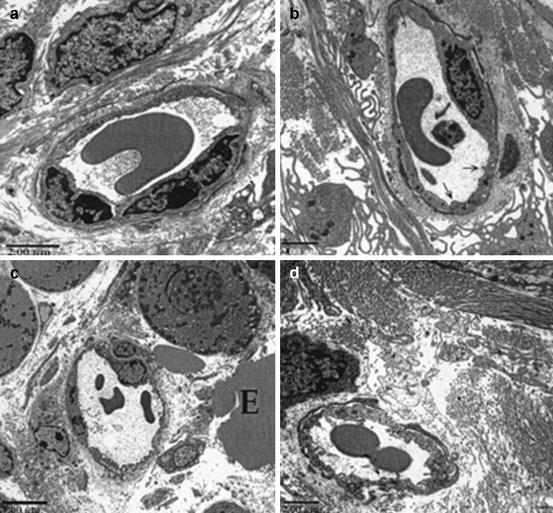

Fig. 6.5
TEM micrographs of dermal capillaries. Capillaries of the NT mouse has thin continuous endothelium with regular and smooth surfaces (a). The capillaries of the IL-4 Tg mouse at BO stage still has continuous endothelium, but are thicker, more irregular and vacuolated. The endothelial cell (EC) nucleus is enlarged with prominent nucleoli, a sign of EC activation in preparation for angiogenesis (b). As disease progresses from EL (c) to LL (d) the capillary endothelium becomes increasingly irregular and vacuolated; ECs become increasingly hypertrophic with large nuclei and nucleoli and enlarged cytoplasm (Reproduced with permission, Agha-Majzoub et al. [39])
Endothelial remodeling is the fundamental process responsible to angiogenesis and we also examined this process by EM in AD. Angiogenic capillaries are distinguished from nonangiogenic capillaries by the presence of hypertrophied endothelial cells that appears activated with organelle rich cytoplasm and a minuscule lumen. In lesional skin of Tg mice, newly formed blood vessels form by intussusception, where interposition of transcapillary pillars allows division of one capillary into two separate capillaries (Fig. 6.6), whereas such observation is absent in NT mice. In Tg-EL and Tg-LL mice, both angiogenic and non-angiogenic capillaries were present.