Acute
Early
Late
Chronic
1–7 days
1–6 weeks
6–12 weeks
>12 weeks
Acute or early postoperative bleeding usually occurs within the first 12–48 h after a surgical procedure but can still manifest as late as 42 days [4, 5]. In gastric sleeve patients, bleeding is mainly related to the long staple line, short gastric vessel pedicles, or trocar sites. In gastric bypass (GBP) patients, bleeding is usually related to an anastomotic or staple-line bleed as well as a stress ulcer or gastritis. The bleeding can be either IL or IA. Other potential causes of IAB in the acute phase can be related as mentioned to trocar sites, surgical dissection sites, or incomplete hemostasis of surgical sites prior to desufflation of the abdominal cavity.
Late and chronic bleeding will usually present more than 42 days after the procedure, and it is mostly related to marginal ulceration, gastritis, or neoplasm. It can occur after any bariatric procedure, but is most commonly seen with GBP patients due to the ulcerogenic nature of the gastrojejunal anastomosis. These ulcers can be seen anywhere at the gastrojejunostomy, in the excluded gastric remnant and duodenum. The etiologies of these ulcers are multifactorial, including local ischemia due to poor blood supply, anastomotic tension, the use of nonabsorbable sutures, increased gastric acidity, Helicobacter pylori infection, tobacco use, and nonsteroidal anti-inflammatory drug (NSAID) therapy [5, 6].
Chronic bleeding in the sleeve gastrectomy (SG) patient is uncommon but can be related to ulcers that can develop but are not as prevalent as in the GBP patient. Cases of chronic bleeding in gastric band (GB) patients have been reported in cases of band erosion, where patients can present with melena or hematemesis. Neoplasms that were not detected preoperatively or arise de novo after the bariatric procedure should be also kept in mind when a patient presents with new onset of signs or symptoms of bleeding [7–10].
Diagnostic Algorithm
Acute and Early Bleeding
Diagnosis of hemorrhage in the acute postoperative period, the first 48 h, is a challenging task. The bariatric population’s body habitus and the new anatomy result in difficult clinical decision-making. Some of the common signs of acute bleeding are increased bloody output if drains are left behind at the time of the primary procedure, abdominal distension, and/or tachycardia, hypotension, and oliguria. Tachycardia has been shown to be a very important indicator of postoperative complications and tends to increase gradually and be cyclical in bariatric surgery patients with postoperative hemorrhage. This cyclical behavior of tachycardia in bleeding episodes differentiates from the septic pattern that ensues in patients with anastomotic leaks where tachycardia stays up and above 120 beats per minute without a cyclical pattern [7] (Fig. 21.1).
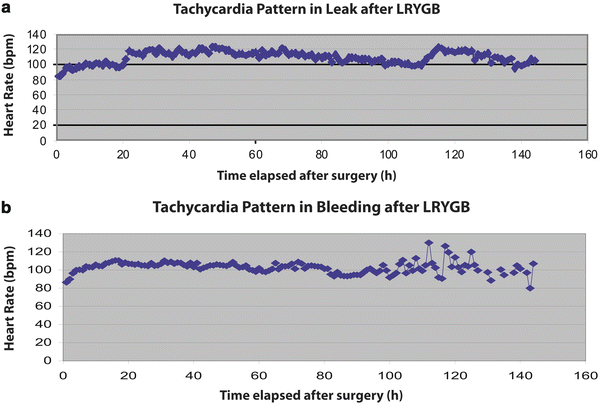

Fig. 21.1
Patterns of tachycardia after (a) leak is sustained >120 bpm, (b) bleeding is cyclical LRYGB laparoscopic roux-en-Y gastric bypass
In the acute setting, one of the biggest challenges is differentiating between IL and IA bleeding, as this will guide further decision-making. Clinical presentation can be helpful when symptoms are typical of IL bleeding like melena and hematemesis. When the symptoms are not present, the use of surgical drains in the immediate postoperative period can guide the surgeon to the appropriate diagnosis as mentioned by Chousleb et al. [2]. If the drains are filling up with bright red blood, it is safe to assume the bleeding is IA.
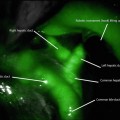
IL bleeding is more commonly seen after laparoscopic Roux-en-Y gastric bypass (LRYGB) due to the many staple lines that are present (gastrojejunostomy, jejunojejunostomy, and gastric remnant) (Fig. 21.2). Heneghan et al. described that 40 % of staple-line bleeds are from the gastric remnant, 30 % from the gastrojejunostomy, and 30 % from the jejunojejunostomy [11]. Nguyen et al. demonstrated that the most common site of bleeding is at the gastrojejunostomy [5].
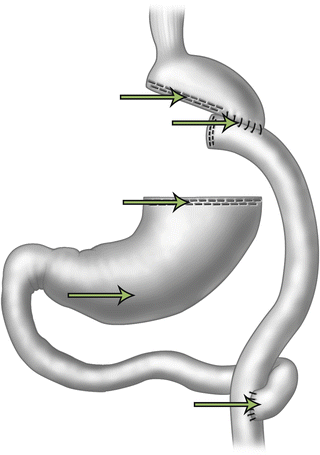

Fig. 21.2
Potential sites for bleeding after gastric bypass
Aside from clinical signs, important diagnostic modalities are upper endoscopy and computed tomography (CT). The latter is only an option when patients are clinically and hemodynamically stable. A CT scan can demonstrate collections/hematomas if bleeding is IA, and sometimes show fluid in the gastric remnant, which is a sign of IL bleeding. CT scan with intravenous contrast can also potentially show, though rare, if there is active bleeding with the presence of a blush/active extravasation. Upper endoscopy is a very useful diagnostic modality since an intervention can be done without delay at the bedside with the patient in the intensive care unit (ICU) to stop the bleeding. Endoscopy should be performed with caution in the immediate postoperative period to avoid disruption of the anastomosis [12–15].
A negative esophagogastroduodenoscopy (EGD) should prompt the surgeon about a possible bleeding site from the jejunojejunostomy or gastric remnant.
Late and Chronic Bleeding
Chronic postoperative bleeding (>30 days) is mostly IL, and often present with clinical signs of upper gastrointestinal bleeding. Upper endoscopy tends to be the diagnostic method of choice [16–18]. Double-balloon enteroscopy can be performed to assess the excluded stomach and duodenum that can be the site of an ulcer or neoplasm. This tends to be a tedious procedure for gastroenterologists because it is easy to get lost in the anatomy, and difficult to access the excluded biliopancreatic limb. A laparoscopic utility gastrotomy can also be performed to gain access to and evaluate the gastric remnant with an endoscope when a double-balloon endoscopy is not possible. If all of the above are negative, new modalities such as capsule endoscopy to identify unusual small bowel pathology can be helpful [19–21].
Treatment Strategies
Acute and Early
Treatment of acute postoperative hemorrhage begins with the clinical assessment. It is important to make sure that the patient is hemodynamically stable. When bleeding is suspected, the patient should be placed in a monitored unit. A Foley catheter should be in place to assure adequate fluid resuscitation, and type and screen and serial blood hemoglobin/hematocrit should be obtained to determine the trend of blood loss and the need for possible blood transfusion. If the patient is clinically stable, further tests such as EGD can be performed, but if there is hemodynamic instability that is not responding to resuscitation, immediate surgical intervention might be required. The majority (>80 %) of acute postoperative bleeding will resolve with conservative management, without the need for another procedure or reoperation [5, 12, 13].
It is very important to differentiate IL bleeding from IA bleeding in the immediate postoperative period. IA bleeding rarely requires a reoperation; the incidence of bleeding has dramatically decreased recently with wide use of staple-line reinforcement during bariatric procedures and with the advancement of hemostatic dissection devices (ultrasonic scalpel/bipolar vessel sealant). In the event that the bleeding does not stop and reoperation is warranted, diagnostic laparoscopy is an excellent option. If during the exploration no obvious source of bleeding is identified, all staple lines should be carefully examined and oversewn if considered appropriate [1].
If IL bleeding is diagnosed, conservative management remains an acceptable option; however, if the patient fails conservative management or is unstable, an invasive approach is the next step. Upper endoscopy is not only an excellent diagnostic modality, but also a good treatment option in patients with acute IL postoperative bleeding. An endoscope can be used to treat bleeding in the gastric pouch of GBP patients and, with the aid of a laparoscopic gastrotomy, has access to the excluded small bowel/stomach. Endoscopic treatment options range from heat to injections to hemostatic clips. In the acute setting, the use of a heat source to stop bleeding is not recommended due to potential disruption of a fresh anastomosis; if possible, clips should be used. Many experts recommend that all endoscopic interventions should be done under laparoscopic observation to monitor for perforation [14]. If bleeding persists despite endoscopic management, oversewing of the affected area will be the definitive option (Fig. 21.3).
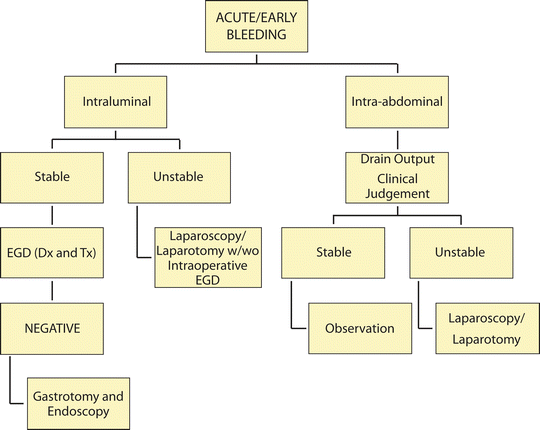

Fig. 21.3
Algorithm for diagnosis/treatment of acute/early bleeding EGD esophagogastroduodenoscopy; Dx and Tx diagnosis and treatment
Late and Chronic Bleeding
When bleeding occurs 42 days or more after the procedure, it is most likely IL and presents with signs and symptoms of an upper or lower gastrointestinal bleed. This bleeding is more likely seen with GBP than other bariatric procedures. Since the most likely cause is ulcer disease, medical treatment with proton pump inhibitors is an acceptable initial treatment method. If bleeding persists despite medical treatment or if there is hemodynamic instability, endoscopy is the best treatment option [18].
Upper endoscopy is the treatment method of choice for bleeding ulcers in the gastric pouch. Rabl et al. reported an 80 % success rate at controlling bleeding in the gastric pouch or the gastrojejunostomy [14]. However, other reports show a success rate of <70 % [15, 16]. When the ulcers are present in the gastric remnant or the excluded duodenum, more advanced endoscopic procedures are required. Double-balloon enteroscopy is a good option; however, the ability to access the biliopancreatic limb and to treat the bleeding is very operator dependent and does not have a high success rate. A variability of limb length among surgeons and adhesions from prior surgeries are some of the potential reasons for an unsuccessful double-balloon enteroscopy. A technically easier but more invasive procedure is a laparoscopic-assisted transgastric endoscopy. Using this method, the endoscopist is directed directly into the gastric remnant and has full access to the rest of the excluded duodenum and small intestine [19–21].
In patients with uncontrolled bleeding or recurrent ulcer disease, a surgical intervention is required. The aim of surgery is to treat the problem and to prevent a recurrence. For this reason, the surgical option of choice for patients with GBP with recurrent ulcers, uncontrolled ulcer bleeding, or multiple ulcer locations is an excision of the gastrojejunal anastomosis with reconstruction of a new gastrojejunal anastomosis or remnant gastrectomy, depending on the site of bleeding. Angiography and embolization have been described for upper gastrointestinal bleeding; however, in GBP patients, if the left gastric artery is embolized, ischemia of the gastric pouch is likely. For this reason, angiography should be reserved for only select patients who are not good surgical candidates [18] (Fig. 21.4




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree