Chapter 10 Physiologic Lipids for Barrier Repair in Dermatology
INTRODUCTION
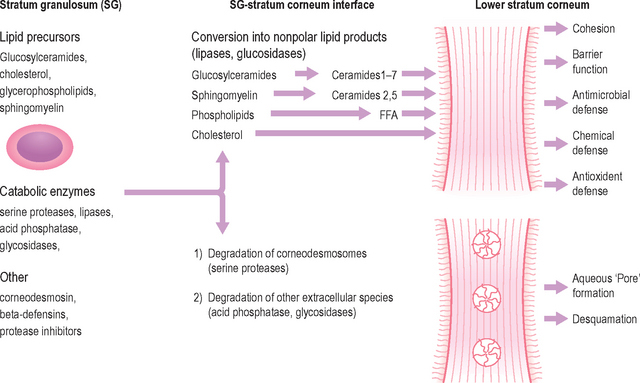
Although the stratum corneum (SC) serves many defensive functions (Table 10.1), none is as important as its ability to prevent excess loss of fluids and electrolytes, i.e. the permeability barrier. Permeability barrier function is mediated by the organization of the extracellular lipids of the SC into a series of parallel lamellar membranes, which mediate not only permeability barrier function, but also additional, key protective functions of the epidermis (Fig. 10.1). The functions of the SC can be further localized to either the cellular (corneocyte) compartment or extracellular matrix (Table 10.1).
Table 10.1 Protective functions of mammalian stratum corneum
| Function | Localization |
| Permeability barrier* | Extracellular |
| Initiation of inflammation (cytokine activation)* | Corneocyte (and granular cell) |
| Cohesion (Integrity) → Desquamation* | Extracellular |
| Antimicrobial barrier (innate immunity)* | Extracellular |
| Mechanical (impact and shear resistance) | Corneocyte |
| Toxic chemical/antigen exclusion | Extracellular |
| Selective absorption | Extracellular |
| Hydration | Corneocyte |
| UV barrier | Corneocyte |
| Psychosensory interface | Unknown |
| Thermal barrier | Unknown |
DYNAMICS OF BARRIER RECOVERY
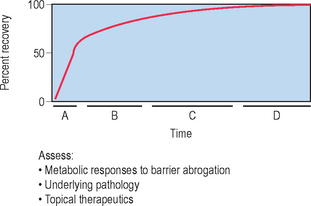
The skin barrier is assaulted frequently in daily life by hot water, detergents, solvents, mechanical trauma, and occupation-related chemicals. If these insults are frequently repeatedly and/or insufficiently repaired, they threaten the organism with desiccation due to accelerated transepidermal water loss (TEWL). To avoid this outcome, the underlying epidermis mounts a coordinated metabolic response, ranging from increased lipid synthesis to accelerated lipid secretion, aimed at rapidly restoring normal function. This response is elicited by any type of barrier insult (e.g. organic solvents, detergents, tape stripping) that depletes the SC of its complement of lipids. Although the total time required for barrier recovery varies according to age, there is an initial, rapid recovery phase that leads to 50–60% recovery in young humans in about 12 hours, with full recovery requiring about 3 days (Fig. 10.2). But in aged humans (> 75 years), complete recovery from comparable insults is prolonged to about 1 week. Restoration of barrier function is accompanied by reaccumulation of lipids, visible with either oil red O staining or Nile red fluorescence, and by the reappearance of membrane structures within the SC interstices, as early as 2 hours after acute disruption. Because artificial restoration of the barrier with vapor-impermeable membranes inhibits barrier recovery, as well as all of the metabolic processes linked to it, the entire metabolic response represents a response that is aimed specifically at restoring normal permeability barrier homeostasis.
CLINICAL APPLICATIONS OF THE CUTANEOUS STRESS TEST
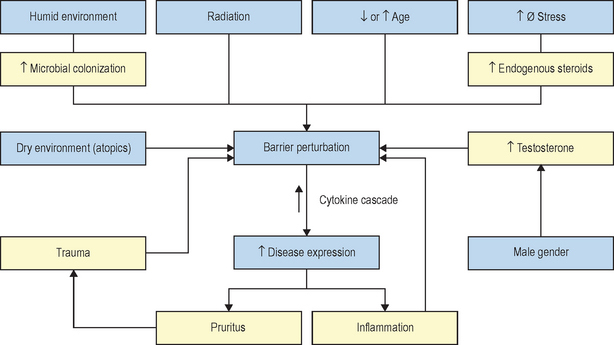
The kinetics of barrier recovery after acute perturbations (also called the ‘cutaneous stress test’) can discern abnormal function or underlying pathology, even when basal parameters are normal (Fig. 10.2), analogous to the cardiac treadmill exam. Indeed, the cutaneous stress test reveals deficient barrier function both in aged and in neonatal skin, despite deceptively normal function in both age groups under basal conditions. Further, it amplifies differences between other ‘normal’ groups (Fig. 10.3):
 testosterone-replete versus testosterone-deficient individuals
testosterone-replete versus testosterone-deficient individuals
 skin exposed to humid versus dry environments
skin exposed to humid versus dry environments
Finally, individuals subjected to increased psychological stress reveal a defect in barrier recovery, explaining the propensity for these factors to exacerbate inflammatory dermatoses, such as psoriasis and atopic dermatitis, which already display barrier abnormalities. We have proposed that these factors further amplify the cytokine cascade that characterizes these disorders (Fig. 10.3).
LIPID COMPOSITION OF LAMELLAR MEMBRANES
The extracellular lipids in the SC derive primarily from the secreted contents of epidermal lamellar bodies (LB), which are enriched in cholesterol, glucosylceramide, phospholipids, as well as their respective hydrolytic enzymes (Fig. 10.1). These lipid-processing enzymes catalyze the extracellular degradation of sphingomyelin and glucosylceramides to ceramides (Cer), and phospholipids to free fatty acids (FFA) that, along with cholesterol (Chol), form the lamellar membranes required for permeability barrier function. Thus, the SC lamellar membrane comprises unique biologic membranes that mediate the barrier because:
 they are extremely hydrophobic
they are extremely hydrophobic
 their total lipid weight percentage in SC (= number of lamellae in the extracellular spaces)
their total lipid weight percentage in SC (= number of lamellae in the extracellular spaces)
 their presence in an equimolar mixture
their presence in an equimolar mixture
 the presence of certain lipids, such as acylceramides, bearing linoleic acid.
the presence of certain lipids, such as acylceramides, bearing linoleic acid.
Together, these characteristics largely explain the barrier properties of the SC.