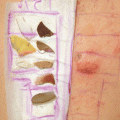
Fig. 7.1
Systemic chronic drug photosensitivity, with symmetrical lesions involving the face and V of the neck and forearms, with protection of the area covered by the wrist watch
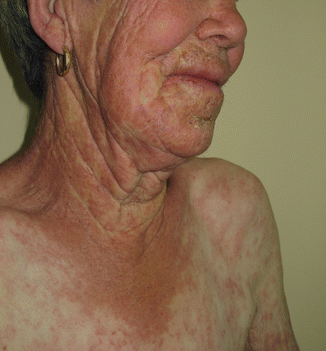
Fig. 7.2
Photoreaction from ingestion of Hypericum perforatum infusion with main lesions on photoexposed areas but eczematous and erythema multiforme-like lesions outside the exposed area, suggesting a concomitant photoallergy
Phototoxicity is more frequent, can occur on first exposure as it needs no previous sensitization, and presents typically as exaggerated sunburn that develops within 24–48 h as erythema, eventually with bullae, usually with sharp limits, and tends to regress with brown hyperpigmentation.
Photoallergy is less frequent than phototoxicity and occurs in a limited number of exposed individuals. Except in a few individuals who are already allergic to a cross-reactive molecule, lesions develop after a longer exposure to the photosensitizer (latency period required for sensitization). Once sensitized, the reaction develops within 24–48 h of exposure and, particularly in PhACD, presents as acute or subacute eczema that begins at the area of application but may extend beyond its limits and, eventually, generalize.
Histopathology shows sunburn cells (apoptotic keratinocytes) and nonspecific inflammation in phototoxicity, whereas in PhACD, an acute eczema with spongiosis and T-cell exocytosis is usually observed, but there is no definite histopathologic distinction between these two patterns (Table 7.1).
Table 7.1
Clinical aspects of photoreactions
Predominant in phototoxicity | Predominant in photoallergy or immune-mediated reactions |
|---|---|
Frequent, can occur first exposure | Rare, needs previous sensitization |
Lesions with sharp limits | Lesions may extend to covered areas |
Exaggerated “sunburn” | Acute vesicular, papular eczema |
Pseudoporphyria | Subacute/chronic eczematous lesions |
Photoonycholysis | Erythema multiforme-like |
Hyperpigmentation | Lichenoid reactions |
Hypopigmentation | Cheilitis |
Telangiectasia | Urticaria on sun-exposed area |
Purpura | Pellagra-like reactions |
Histology – sunburn cells | Histology – spongiosis, lymphocyte exocytosis |
Quick regression | Possible persistence/recurrence and cross-reactions |
Increase in actinic keratosis and nonmelanoma skin cancer in the long term | Possible subacute or chronic cutaneous lupus erythematosus |
7.3 Main Causes of Photoreactions
A phototoxic dermatitis occurs frequently after contact with plants rich in furocoumarins (Moracea, e.g., Ficus carica, or Rutacea, e.g., Ruta graveolens, and citrus fruit peels, particularly lime, Citrus aurantifolia). It presents as linear lesions with non-pruritic erythema and bullae, in the acute stage, followed by long-lasting brown hyperpigmentation streaks (Fig. 7.3). Ingestion of infusions of these plants, like Hypericum perforatum used as folk medicine to treat depression (Fig. 7.2), can also cause systemic photoreactions [5].
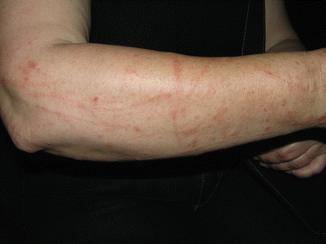

Fig. 7.3
Linear inflammatory and pigmented lesions from phytophotodermatitis from Ruta graveolens
Drugs are the main cause of systemic photosensitivity, mostly dependent on phototoxicity, even though other mechanisms (increased porphyrins levels, as with vemurafenib) [6, 7] and photoallergy also have to be considered (as in photoallergy to the nonsteroidal anti-inflammatory drug (NSAID) piroxicam). Cutaneous lesions in systemic drug photosensitivity involve photoexposed areas mostly in a symmetric distribution and present mainly as eczematous lesions or exaggerated sunburn, or also as pseudoporphyria, simulating porphyria cutanea tarda (naproxen, voriconazole, celecoxib), photoonycholysis (tetracyclines), hyperpigmentation (amiodarone), vitiligo-like lesions (flutamide), telangiectasia (ciprofloxacin), or subacute cutaneous lupus erythematosus (terbinafine, thiazides). Accelerated skin photoaging and an increase in precancerous skin lesions and skin cancers, mostly nonmelanoma skin cancer, are being described as delayed manifestations of exposure to photoactive drugs (voriconazole, fluoroquinolones) [7–11].
Many classic topical photosensitizers have been removed from the European market and now seldom cause PhACD – halogenated salicylanilides used in disinfectant soaps, musk ambrette and bergamot oil used as perfumes, olaquindox an antibiotic used as a pig feeder, and PABA (para-aminobenzoic acid) and isopropyl-dibenzoylmethane used in sunscreens. At present, main causes of PhACD are the UV filters and topical NSAID, with predominance for the latter in Southern European countries [12–16]. UV filters are frequently used for individual photoprotection but also to prevent degradation of the products and increase their shelf life. Therefore, apart from sunscreens, where they are present in higher concentrations and number, UV filters are also present in moisturizing, anti-wrinkle, and facial creams and other make-up (e.g., lipstick), nail varnish, shampoos and other cleansing products, and hair products [17]. At present, the main chemicals responsible for PhACD or photoaggravated ACD are oxybenzone or benzophenone 3, octocrylene, butylmethoxydibenzoylmethane, and cinnamates [13, 14, 16, 18]. Newer UV filters, like Mexoryl SX® (terephthalylidene dicamphor sulfonic acid), Tinosorb M® (methylene bis-benzotriazolyl tetramethylbutylphenol or bisoctrizole), and Tinosorb S® (bis-ethylhexyloxyphenol methoxyphenyl triazine), seldom cause PhACD. They are mostly photostable molecules used in mixtures of sunscreens that also photostabilize older photo labile UV filters, like the dibenzoylmethanes. This may explain why, although the use of products containing UV filters is certainly growing, there is no parallel increase in PhACD from these chemicals. Some of them can also cause ACD, particularly Tinosorb M®, due to the surfactant decylglucoside that is used to solubilize the active molecule of bisoctrizole [19, 20].
In most recent studies on photopatch testing, NSAIDs are the main cause of positive photopatch tests, with ketoprofen and related drugs (piketoprofen and dexketoprofen) or cross-reactive substances as the main responsible [16]. Ketoprofen used in gel, and more recently also in transdermal patches, often induces severe forms of PhACD (acute eczema, erysipela-like reactions, erythema multiforme) (Fig. 7.4) that occur very soon after initiating treatment and may persist or recur on sun exposure with no apparent further contact with the drug. This may be explained because, after topical exposure, the drug persists in the epidermis for more than 2 weeks [21]. Also, there are cases of ectopic (at sites distant from the original application), connubial, or “by proxy” contact dermatitis due to contact with the skin/hands contaminated by ketoprofen gel or by contact with contaminated objects, namely, from clothes that retain ketoprofen even after being washed [22–27].
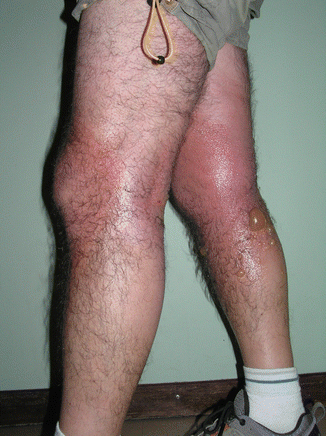

Fig. 7.4
Severe PhACD from a topical NSAID, sparing the thighs under the clothing
PhACD to ketoprofen is associated with frequent cross-reactive photopatch test reactions to other arylpropionic NSAIDs (tiaprofenic acid and suprofen); benzophenone UV filters, mainly oxybenzone, and the systemic hypolipemic agent, fenofibrate, that induces systemic photosensitivity. Positive photopatch tests to the UV filter octocrylene and patch tests to fragrance mix I and to its constituent cinnamic alcohol are also associated with PhACD to ketoprofen [13, 28–33].
Piroxicam is another NSAID that causes both topical and systemic photoallergy, mostly after previous contact sensitization to thiomersal and its moiety thiosalicylic acid, as photoproducts of piroxicam are chemically similar to these contact allergens [13, 34].
Benzydamine, a topical NSAID used mainly in mouth washes or genital soaps, can cause PhACD, that in the first case presents as cheilitis and chin dermatitis and in the latter involves the dorsum of the hands [3, 35].
Phenothiazine derivatives used in some few European countries as topical antihistamines (promethazine, isothipendyl chlorhydrate) or muscle relaxants (chlorproéthazine) or chlorpromazine whose pills are smashed by caregivers to give disabled patients cause frequent PhACD in countries where they still are available [13, 36–39] (Table 7.2).
Table 7.2
Main exogenous agents causing photoreactions
1. UV filters |
Benzophenones: oxybenzone, sulizobenzone, mexenone |
Dibenzoylmethanes: butyl methoxydibenzoylmethane |
Cinnamates: isoamyl-p-methoxycinnamate, ethylhexyl methoxycinnamate, octocrylene, drometrizole trisiloxane |
4-methylbenzylidene camphor, phenylbenzimidazole sulfonic acid |
2. Plants (main families in Europe) |
Umbelliferae: Ammi majus; Apium graveolens (celery) |
Pastinaca sativa (parsnip); Petroselinum crispum (parsley) |
Heracleum mantegazzianum (giant hogweed) |
Rutacea: Citrus spp., Citrus aurantica v. bergamia (bergamot) |
Citrus aurantifolia (lime); Citrus limon (lemon) |
Ruta graveolens (common rue); Dictamus albus (burning bush) |
Moracea: Ficus carica (fig) |
3. Drugs |
a. Antimicrobials |
Doxycycline, minocycline, sulfamethoxazole |
Fluoroquinolones (lomefloxacinb, ciprofloxacinb) |
Voriconazole, griseofulvin, efavirenz |
b. Nonsteroidal anti–inflammatory drugs (NSAIDs) |
Ketoprofena, tiaprofenic acidb, suprofen, carprofen |
Piroxicamc, benzydamineb, etofenamateb |
c. Other drugs |
Chlorpromazine, promethazineb, chlorproethazine |
Amiodarone, furosemide, and thiazide diuretics |
Paclitaxel, 5-fluorouracil, dacarbazine, vemurafenib |
Fenofibrate, flutamide, sulfonylureas |
4. “Historical” photosensitizers d |
Perfumes: musk ambrette and bergamot oil |
Halogenated salicylanilides |
Sunscreens: isopropyl-dibenzoylmethane, PABA |
Antibiotics: Olaquindox |
7.4 Whom, When, and How to Test Patients
Photopatch testing is indicated to study PhACD and, in selected cases, can be helpful in systemic drug photosensitivity [34, 40), but it is not recommended in typical phototoxic reactions. Therefore, photopatch testing should be performed in all individuals, including children, with dermatitis on photoexposed areas, dermatitis aggravated by UV exposure, sunscreen intolerance, or exposure to NSAID [16, 18]. In patients with chronic photosensitivity (chronic actinic dermatitis, polymorphic light eruption, cutaneous lupus erythematosus), photopatch testing may be important to exclude a concomitant PhACD, e.g., to a UV filter. In these individuals with a lowered threshold of UV sensitivity, phototesting is usually performed along with photopatch testing, in order to program the adequate UV doses for patch test irradiation [16, 41].
Photopatch testing should be performed, whenever possible, when there are no active lesions or, at least, when the back is clear and when the patient has withdrawn immunosuppressive drugs. If not possible (solid-organ-transplanted patients), interpretation must be cautious, as false-negative results may occur. Photopatch testing should be postponed after sunburn or significant sun exposure on the back, after local use of corticosteroids, and in patients on potential photoactive drugs.
7.5 Photopatch Testing: Technique and Requirements
For performing photopatch testing, apart from material common to contact allergy clinics (allergens and tests chambers), a UV source is necessary. Any broadband UVA source (320–400 nm) with a photometer to quantify UV light delivered to the skin, e.g., a cabin with UVA lamps for PUVA therapy, can be used for UV irradiation.
Recently, ESCD (European Society of Contact Dermatitis and Cutaneous Allergy) and ESP (European Society of Photodermatology) agreed on a recommended European baseline photopatch test series and an extended series including mostly UV filters, NSAIDs, and topical drugs (Table 7.3




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree