Fig. 30.1
Dual action mechanisms for phototherapy-apoptosis and immune suppression
30.3.1 Regulatory T Cells (Treg) Induction
Narrowband UVB therapy generally induces a relatively long remission period of approximately 4–6 months in patients with psoriasis. The induction of apoptosis, however, might be only partially responsible for this relatively long remission period. The role of Treg should also be considered, as narrowband UVB radiation suppresses local and systemic immune responses in a model of contact hypersensitivity [31]. Tregs have an immune regulatory function and play a key role in peripheral tolerance [27, 30]. Peripheral T cells from patients undergoing UVB phototherapy exhibit a CD4+CD25+ T-cell profile [15]. Induction of Treg cells following UV irradiation is associated with UV-induced DNA damage, which induces Langerhans cells to move from the skin into the draining lymph nodes, and IL-12 can induce DNA repair and limit the number of UV-damaged Langerhans cells in the draining lymph nodes [28]. It is thus possible that UV-induced DNA damage alters cutaneous antigen-presenting cells and enhances their ability to activate Treg cells. UV irradiation increases the proportion of fluorescein isothiocyanate-bearing dendritic cells within the draining lymph nodes [19] that exhibit deficient maturation and deficient T-cell priming [18]. UV irradiation, however, also promotes the generation of CD4+CD25+Foxp3+ T cells within the draining lymph nodes following immunization [29]. Among these CD4+CD25+Foxp3+ T cells are cells with an antigen-specific regulatory function in vivo [5]. The findings from these experiments suggest that CD4+CD25+Foxp3+ T cells, that is, Treg, are responsible for the immunosuppressive effect.
30.3.2 Dysfunctional Treg in Psoriasis Is Restored by Phototherapy
In psoriasis , there is a functional defect in Treg suppressor activity that is not associated with a decrease in the number of CD25+ Treg in the peripheral blood [34]. The quantity and quality of Treg might be related to the pathogenesis of psoriasis. In our previous study, there was no difference in the percentage of Treg between psoriasis patients and healthy controls [26]. In another report, the frequencies of circulating Treg were higher in patients with severe psoriasis than in normal controls [40]. We analyzed the number of Treg in 68 patients and 20 controls. Mean Treg levels were not significantly higher in patients than in normal healthy controls. Based on our two independent studies, the number of Treg in psoriasis patients is similar to that in healthy controls [3].
We demonstrated that bath-psoralen UVA (bath-PUVA) therapy induced circulating Foxp3+ Treg in 10 patients with psoriasis who were first treated with phototherapy [26]. We analyzed the Treg levels in 68 patients before and after phototherapy. Although Treg levels were not increased by phototherapy in any of the 68 patients, Treg levels in patients with less than 4.07 % Treg, defined as the mean of the controls, were significantly increased. We further assessed the Treg function before and after phototherapy. To confirm the previous report that psoriasis patients have dysfunctional Treg, we assayed the Treg function. The Treg functional ratio was significantly lower in psoriasis patients than in age-matched controls. The Treg functional ratio was significantly increased and the Treg function was restored to almost normal levels. These findings suggest that successful phototherapy not only increased the Treg number, but also restored the Treg function [3].
30.3.3 Imbalance of Th17 Cells and Treg Is Normalized by Phototherapy
Th17 cells producing IL-17 , IL-22 , and tumor necrosis factor-α are pathogenically relevant to psoriasis. The imbalance of Th17 cells and Treg is thought to contribute to the pathogenesis of psoriasis. In a clinical study, 14 patients with moderate to severe psoriasis were treated with narrowband UVB. Narrowband UVB suppressed the IL-23/IL-17 pathways, including IL-12/23p40, IL-23p19, IL-17, and IL-22, in normalized plaques, but not in nonresponsive plaques [9]. In another study, gene expression profiling was performed using epidermal RNA from lesional and nonlesional skin undergoing narrowband UVB phototherapy. The Th17 pathway was downregulated during narrowband UVB phototherapy in psoriatic epidermis [24]. In our study, Th17 levels were compared before and after narrowband UVB (n = 18) and bath-PUVA (n = 50). Th17 levels were not decreased by narrowband UVB and bath-PUVA in the 68 patients. Patients with more than 3.01 % Th17, defined as the mean +1 SD of controls, were defined as the high-Th17 population. Th17 levels in the high-Th17 population were significantly reduced by phototherapy. Our findings indicated that successful phototherapy restored Th17 levels back to normal levels in the high-Th17 population [3].
Serum levels of both IL-17 and IL-22 were significantly increased in psoriasis patients compared to healthy volunteers. Phototherapy significantly decreased serum levels of both IL-17 and IL-22 in psoriasis patients. Furthermore, the percent reduction of the Psoriasis Area and Severity Index was correlated with serum levels of IL-6 , but not IL-17 or IL-22, before phototherapy, suggesting that psoriasis patients with high serum IL-6 levels are more susceptible to phototherapy. Based on this study, phototherapy might suppress serum IL-17 and IL-22 levels by inhibiting the IL-6 induced generation of Th17 [14].
These findings indicate that phototherapy induces a decrease in Th17 and an increase in Treg in the peripheral blood of patients with psoriasis, thereby resolving the TH17 and Treg imbalance in these patients (Fig. 30.2). Treg induction is a target for phototherapeutic efficacy. The detailed mechanisms of Treg induction/restoration, however, remain unclear. Moreover, it is necessary to investigate whether Treg proliferates in lymph nodes or lesional skin. Monitoring Treg function might help to establish a more effective phototherapy regimen in a clinical setting.
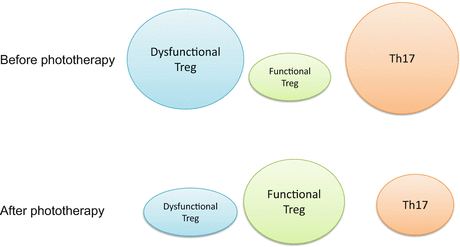

Fig. 30.2
Imbalance of Treg and TH17 in the pathogenesis of psoriasis. After phototherapy, Treg function is restored and Th17 levels in the circulating blood are normalized
30.4 Practical Phototherapy for Atopic Dermatitis: Narrowband UVB, PUVA, and UVA-1
Phototherapy is used for refractory skin disease when topical steroid treatment is not effective. PUVA therapy has been used effectively for general-purpose treatment for 30 years throughout the world. Psoralen is administered orally, topically, or by bath. Due to its efficacy and safety, bath-PUVA therapy is a standard treatment among phototherapies. Narrowband UVB (311 nm) therapy and UVA-1 (340–400 nm) therapy are also applied for the treatment of refractory skin diseases. These phototherapies employ selective wavelengths to reduce the risk of carcinogenicity and increase their efficacy [12]. For example, the first line of treatment for atopic dermatitis comprises topical steroid and moisturizer treatment, as well as instruction regarding lifestyle influences and the removal of any known causes of skin irritation. Phototherapy is used as a second-line therapy when adverse reactions occur and control is difficult with topical steroid treatment. Phototherapy can result in long-term remission, and it may also be possible to reduce the class of steroid used. In addition, with phototherapy, the topical steroid dosage can be reduced even in cases of resistance to topical therapy alone. For moderate and chronic cases, narrowband UVB can be combined with topical steroid treatment [11].
UVA-1 phototherapy uses a wavelength of 340–400 nm. The exposure dose can be relatively high, leading to erythema, due to removal of the short wavelength, UVA-2 (320–340 nm). For acute and severe cases, UVA-1 phototherapy can be used as monotherapy. UVA-1 therapy may be as effective or even more effective than PUVA therapy for the treatment of atopic dermatitis.
Narrowband UVB phototherapy has proved to be an ideal modality for maintenance therapy once high-dose UVA-1 has been used in the initial phase of management of an acute severe exacerbation of atopic dermatitis [11]. If high-dose UVA-1 therapy is not available, severe atopic dermatitis should be controlled prior to the start of phototherapy by aggressive topical steroid therapy or systemic immunosuppressive modalities, such as cyclosporin A.
30.5 Immunologic Effects of UVA-1 Phototherapy
Photoimmunologic mechanisms responsible for the therapeutic effectiveness of UVA-1 therapy in atopic dermatitis have been investigated. Phototherapy of atopic dermatitis employing UVA-1 (340–400 nm), which effectively penetrates the dermal layers of human skin and thus has the potential to affect intradermal T cells directly, is superior to short-wavelength UVB radiation [10], which is almost exclusively absorbed by the epidermis. Accordingly, successful UVA-1 therapy of atopic dermatitis is associated with a significant downregulation of the in situ expression of T-helper-cell–derived cytokines as well as a significant reduction in the number of intradermal CD4+ T cells [8]. We demonstrated that UVA-1 phototherapy induces apoptosis in T-helper cells present in the eczematous skin of atopic dermatitis patients [20]. UVA-1 irradiation-induced apoptosis in human atopen-specific T-helper cells from atopic dermatitis was mediated by the FAS/FAS-ligand system and induced through the generation of singlet oxygen [20]. To assess whether UVA-1 phototherapy of atopic dermatitis patients induces apoptosis in skin-infiltrating T-helper cells, sequential biopsies were obtained from the eczematous skin of five patients before and after UVA-1 radiation. Before therapy, numerous CD4+ cells were present intradermally in lesional skin. Already after the first UVA-1 radiation exposure, CD4+ apoptotic cells were detected. Subsequent UVA-1 treatments led to further increases in the number of apoptotic cells, and a decrease in the total number of CD4+ cells. After 10 exposures, the total number of intradermally located CD4+ T-cells was significantly diminished, and the remaining cells almost all showed signs of apoptosis.
The mechanisms by which UVA-1 and UVB irradiation induce T-cell apoptosis differ markedly. In general, UVA-1 irradiation can cause preprogrammed cell death (early apoptosis), which is protein synthesis-independent, as well as programmed cell death (late apoptosis), which requires de novo protein synthesis [6]. In contrast, UVB irradiation (and also PUVA treatment) exclusively induces late apoptosis [7]. UVA-1 radiation causes both early and late apoptosis, with UVA-l-R-induced singlet oxygen generation being the initiating event, leading to T-cell apoptosis [20]. Singlet oxygen production induces the expression of Fas-ligand molecules on the surface of UVA-1–irradiated T cells. The key role of singlet oxygen in eliciting early apoptosis in human T cells has been corroborated in an independent study using Jurkat cells. UVA-1 radiation/singlet oxygen is postulated to act on the mitochondria and induce Jurkat cell apoptosis by opening the megachannels and by decreasing the mitochondrial membrane potential [7]. The capacity to induce early apoptosis in mammalian cells seems to be highly specific for UVA-1 radiation and singlet oxygen. From a phototherapeutic point of view, this qualitative difference suggests that UVA-1 phototherapy is superior to UVB or PUVA therapy for skin diseases in which the induction of apoptosis in pathogenically relevant cells is of critical importance. The unique properties of UVA-1 radiation have also stimulated interest in its therapeutic use for patients with cutaneous T-cell lymphoma [23]. In vitro studies indicate that malignant T cells are exquisitely sensitive to UVA-1 radiation-induced apoptosis [39].
Immunohistochemical studies of biopsy specimens obtained from patients with atopic dermatitis undergoing UVA-1 therapy indicate that, in addition to T cells and keratinocytes, epidermal Langerhans cells and dermal mast cells represent target cells for UVA-1 radiation. UVA-1 therapy, in contrast to UVA/UVB therapy, reduces not only the relative number of immunoglobulin E-bearing Langerhans cells in the epidermis, but also the number of dermal CDla+ Langerhans cells and mast cells. The latter observation prompted the use of UVA-1 therapy in the treatment of patients with urticaria pigmentosa, in which immediate and long-lasting remissions from cutaneous and systemic symptoms are achieved [33].
30.6 Immunologic Effects of 308-nm Excimer Light Therapy
Excimer light (308 nm) therapy is another option for phototherapy, particularly for the treatment of psoriasis. This method can effectively target the affected area (targeting therapy) and prevent unwarranted exposure of normal skin [1, 2]. Moreover, compared to narrowband UVB, excimer light effectively treats resistant and localized psoriasis lesions with fewer treatments and a lower cumulative UVB dose [1]. The mean number of treatments using an excimer lamp is approximately one-third that of using narrowband UVB phototherapy [9]. Although shorter wavelengths may damage DNA and present greater risks of erythema and carcinogenesis, such long-term risks might be outweighed by the comparative advantages of fewer necessary treatments and lower cumulative UVB doses.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree