Fig. 2.1
Periodontal tissues: the cementum, alveolar bone, periodontal ligament, and gingiva
Shaped during teeth’s formation and development, these structures may be modified during a subject’s life, leaving their “pristine” characteristics, as result of several inherent local, environmental, and systemic conditions. For instance, parafunctional habits/heavy occlusal forces may promote a reinforcement of the bone trabeculae and the deposition of cellular cementum at the root apex (Fig. 2.2a) [2, 3], periodontitis promotes a resorption of alveolar bone and loss of periodontal ligament (Fig. 2.2b–e) [4, 5], and tobacco smoking may decrease peripheral vascularization and increase keratinization of oral gingival epithelium [6]. Also, even in well-maintained subjects who practiced oral hygiene and returned periodically for maintenance care appointments, slight to moderate incidence of periodontal destruction (0.02–0.1 mm/year) may increase with age, especially between 50 and 60 years of age [7]. Apart of any “positive” or “negative” impact on the periodontium, it is of paramount importance to consider that the conditions of periodontal structures surrounding a natural tooth are directly associated to the patient’s quality of life by maintaining the ability to chew, digest food, and smile. Conversely, the clinical response of an individual tooth to periodontal treatment over time may not be easy to be predicted precisely, particularly if the tooth has been exposed to periodontal disease and anatomic and/or traumatic conditions [5, 8–11].
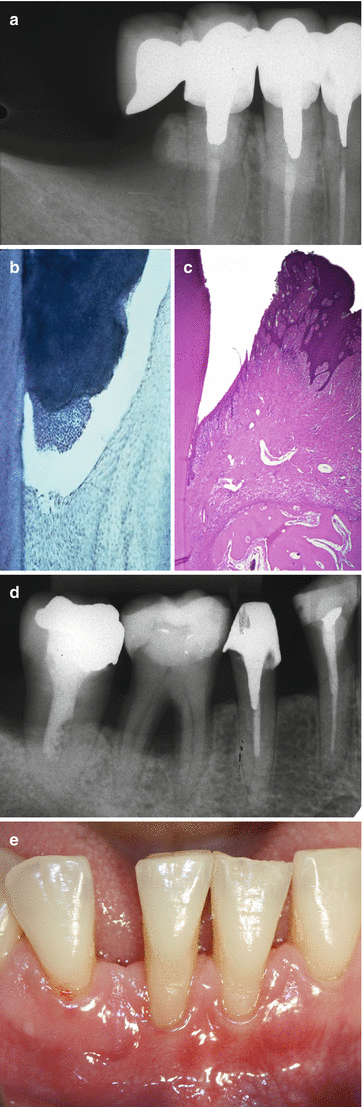

Fig. 2.2
Increase of the cementum and trabecular bone in response to occlusal forces (bony exostosis) (a); periodontitis (b–e)
In the field of periodontics/periodontology, when a patient presents discrepancies in soft tissue morphology, osseous architecture, tooth morphology (e.g., color alteration and shape), and positioning, aesthetical and/or functional treatment approaches may optimize the “pink” and “white” aesthetics concomitantly. Especially within aesthetic areas, the treatment of soft/hard tissue deformities represents an important challenge for the dental clinician, since it may involve a complex, multidisciplinary decision-making process for the concomitant accomplishment of health and harmony between dental and periodontal tissues. For such cases, the balance between function and aesthetics will be dependent on the clinician’s skills and knowledge on tissue anatomy and morphology. Thus, the actual treatment paradigm must be associated with composed functional (e.g., reestablishment of health and proper occlusion) and aesthetical/cosmetic approaches, or in other words, the treatment planning of aesthetic areas must lead to satisfactory clinical and aesthetic long-term results, by achieving a pleasant and harmonious smile [8–11].
2.2 The Anatomy of Soft Tissues Surrounding Human Teeth: The Mucogingival Complex
“The mucogingival complex” is formed by the portion of the oral mucosa that covers the alveolar process including the gingiva (keratinized tissue) and the adjacent alveolar mucosa [1]. Clinically, the gingiva (“the fibrous investing tissue, covered by keratinised epithelium, that immediately surrounds a tooth and is contiguous with its periodontal ligament and with the mucosal tissues of the mouth”) [1] can be found around the cervical portion of the teeth and is characterized by (a) a pink and opaque color, (b) scalloped appearance/contour, (c) a stippling texture that reminds an “orange peel,” and (d) firm consistence, (e) may present melanic pigmentation, and (f) may vary in width, and (g) it usually assumes its definitive shape and texture following the eruption of permanent teeth (Fig. 2.3) [12, 13].
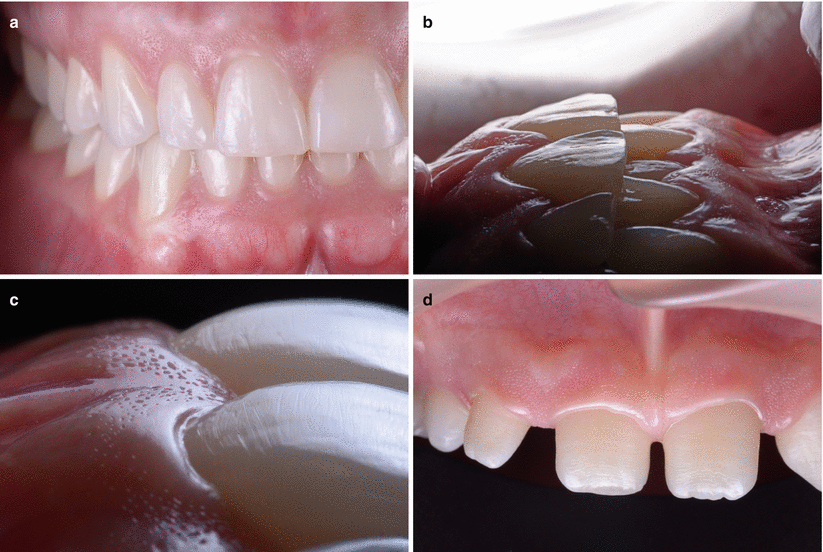

Fig. 2.3
Clinical characteristics of the gingiva
In terms of clinical anatomic limits, the gingiva is delimited by the free/marginal gingiva (“that part of the gingiva that surrounds the tooth and is not directly attached to the tooth surface/the most coronal portion of the gingiva that forms the wall of the gingival crevice in health”) [1] and the attached gingiva (“the portion of the gingiva that is firm, dense, stippled, and tightly bound to the underlying periosteum, tooth, and bone”). The free/marginal gingiva includes the interdental papilla (“that portion of the gingiva that occupies the interproximal spaces [the interdental extension of the gingiva]”) [1] and the col (“a valley-like depression of the interdental gingiva that connects facial and lingual papillae and conforms to the shape of the interproximal contact area”) [1]. Within anterior interproximal sites, the interdental papilla assumes a triangular shape, whereas at the posterior teeth these are more “smooth-edged” given the impression that the posterior papilla is formed by two parts, one buccal and one lingual separated by the col area [13, 14]. Usually, the free/marginal gingiva extends to the gingival groove (“a shallow, V-shaped groove or indentation that is closely associated with the apical extent of free gingiva and runs parallel to the margin of the gingiva”) [1] where it “connects” to the attached gingiva [13, 14]. Regarding the attached gingiva it extends from the apical limit of the free/marginal gingiva to the mucogingival junction, its width may vary between the anterior and posterior teeth, and it may increase with age (Fig. 2.4) [13, 14].
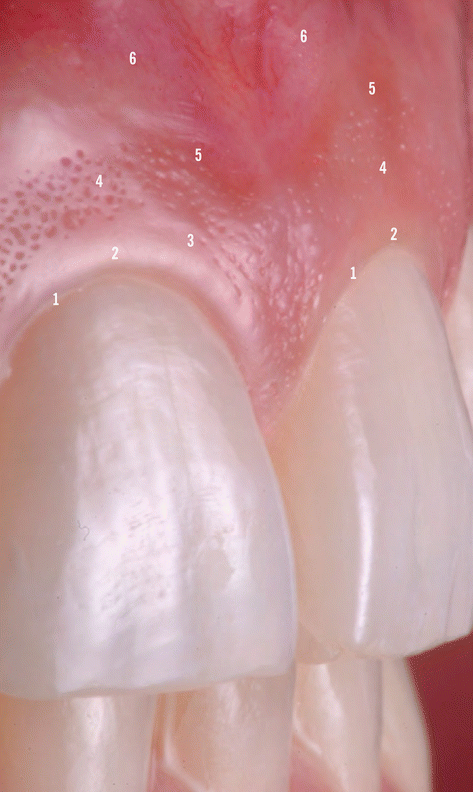

Fig. 2.4
Anatomic limits of the gingiva. 1 Gingival margin, 2 free gingival margin, 3 gingival groove, 4 attached gingiva, 5 mucogingival junction, 6 alveolar mucosa
Microscopically, the gingiva is formed by three epithelial (oral, oral sulcular/crevicular, and junctional) and one connective tissue layer (Fig. 2.5). With the assistance of biopsy samples of human teeth sectioned in a buccal–lingual plane, it can be seen that the buccal gingiva facing the oral cavity presents a layer of keratinized stratified squamous epithelium denominated “oral epithelium” that extends from the mucogingival junction to the gingival margin (Fig. 2.6). This epithelium is formed by four layers/stratums (Fig. 2.7):
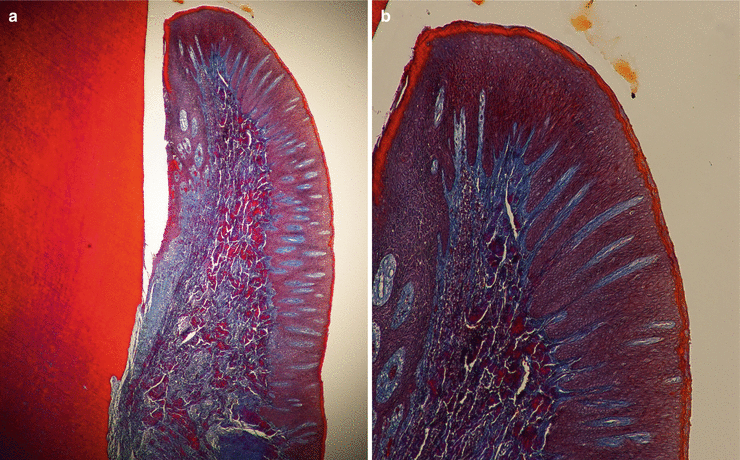
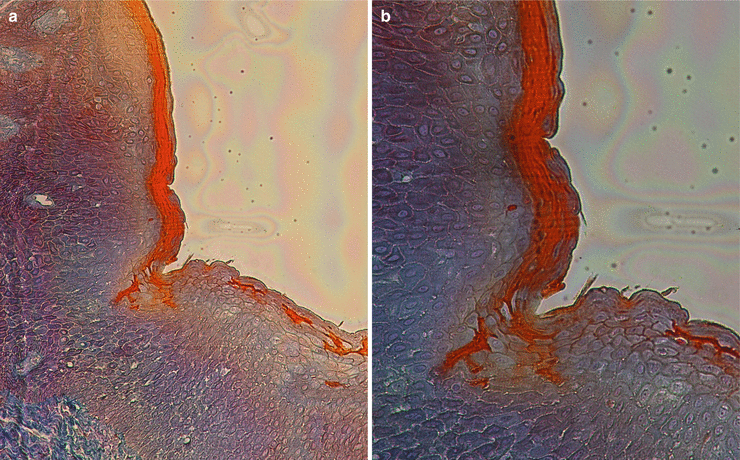
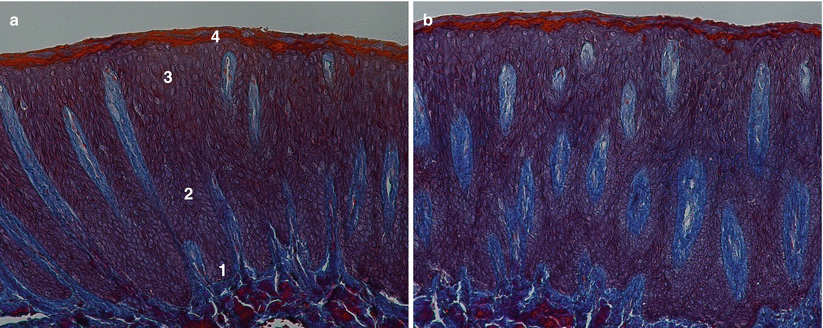

Fig. 2.5
The oral epitheliums (40×) (a) and (100×) (b)

Fig. 2.6
Mucogingival junction (100×) (a) and (200×) (b)

Fig. 2.7
Epithelial layers (1 basale, 2 spinosum, 3 granulosum, 4 0 corneum (200×))
Basale: Formed by rounded and small proliferative cells, known as keratinocytes. These mother cells multiply constantly forming new cells (daughter cells) that will follow through the other three stratums until reaching the corneum one [13, 15]. Also, they are in contact with the basement membrane (the membrane that separates the connective tissue of the lamina propria from the gingival epithelium) [13, 15].
Spinosum: Considered to be the thickest stratum of all, it is formed by large polyhedral spinous-appearing cells [13, 15].
Granulosum: This layer received this name because of the presence of flattened-shaped cells that present within their cytoplasm the presence of dense cytoplasmic keratohyalin granules (this protein provides the “grainy aspect” of this stratum and leads to nuclei and organelle disintegration) [13, 15].
Within the oral epithelium, different types of cells may be found, such as melanocytes (cells that produce the dark, amorphous pigment of the skin, hair, and the choroid coat of the eye and that give the darkish color of the gingiva – Fig. 2.8) [1], Langerhans (defense cells originated of macrophages), and Merkel (sensorial/tactile) cells [13, 15].
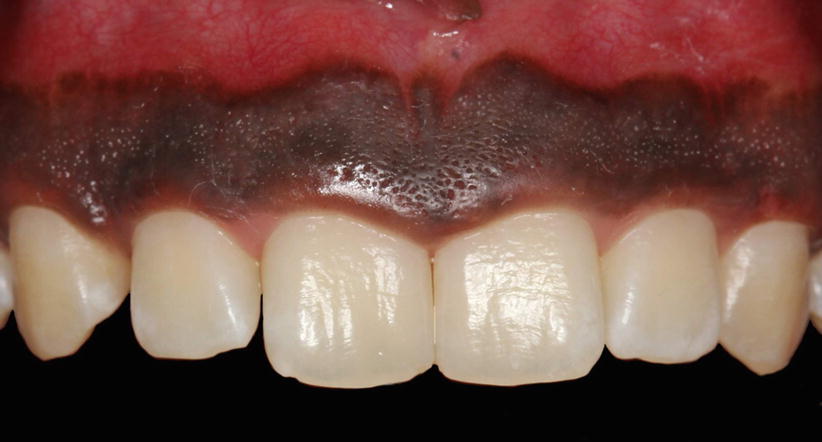

Fig. 2.8
Pigmented gingiva (melanocytes of the oral epithelium)
At the gingival margin, the oral epithelium “continues” through the interior of the gingival sulcus/crevice (i.e., the shallow fissure between the marginal gingiva and the enamel or cementum) [1], and there it becomes the oral sulcular/crevicular epithelium, a nonkeratinized stratified squamous epithelium formed by three layers (basale, spinosum, and granulosum) [13–15]. Despite being described as “nonkeratinized,” there has been evidence of areas/portions of keratinization mainly close to the entrance of the crevice (Fig. 2.9). Additionally, in the presence of gingival health, it will allow the formation of a gingival sulcus/crevice of 0.69 mm that extends from the gingival margin to the bottom of the crevice all around the tooth [16].
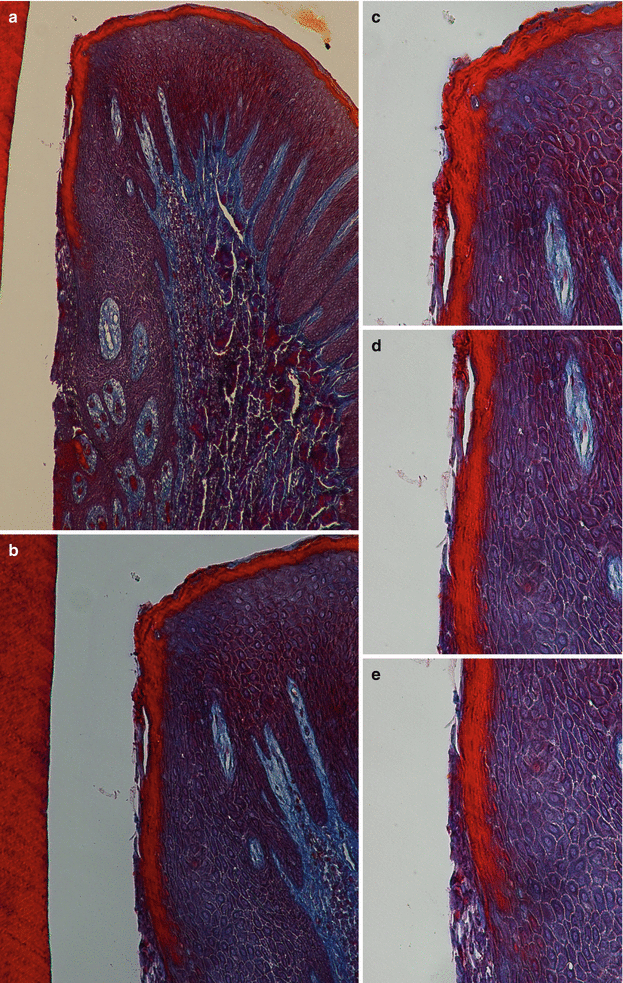

Fig. 2.9
Sulcular/crevicular epithelium (100×) (a, b) and (200×) (c–e)
The bottom of the gingival crevice separates the sulcular of the junctional epithelium, which is defined as “a single or multiple layer of nonkeratinizing cells adhering to the tooth surface at the base of the gingival crevice” [1]. It is formed by 15–30 parallel layer cells at its most coronal portion adjacent to the oral sulcular/crevicular epithelium to just a few cells at its most apical position [15]. Despite presenting just two strata (basale and suprabasale), the junctional epithelium is characterized by its capacity of sealing the internal or the external environment and by its high capacity of cellular turnover (as provided by its basal layer) and bigger intercellular spaces than the other two epitheliums (Fig. 2.10) [13, 15]. These features provide the ability not only of attachment, but of protection because of cell desquamation at its most coronal portion (i.e., close to the base of the gingival sulcus/crevice) and permeability to polymorphonuclear neutrophils (which are quite evident) [13, 15].
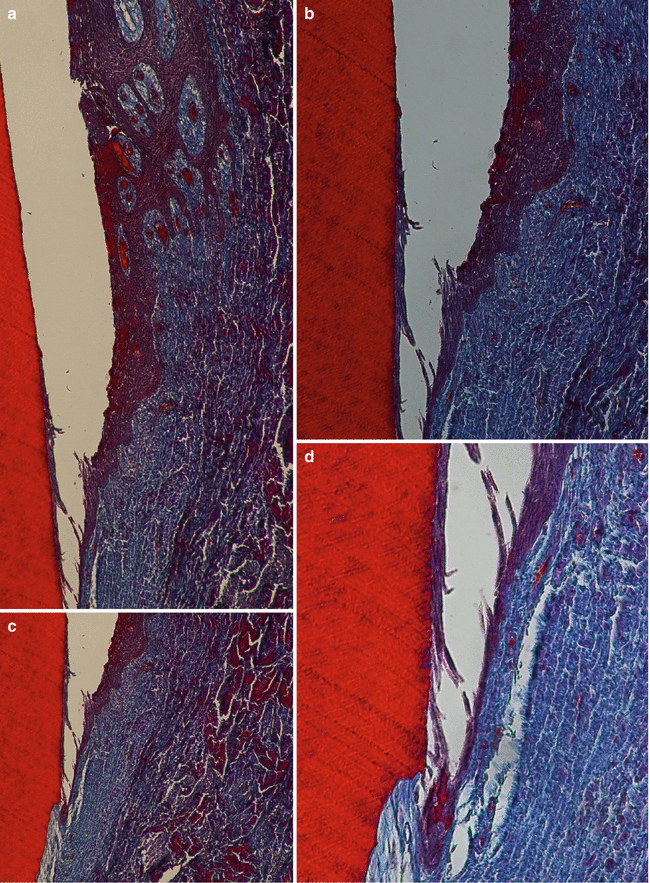

Fig. 2.10
The junctional epithelium (transition between the sulcular epithelium and the junctional epithelium). Note to the readers: the specimens presented in this chapter are derived from a clinical study on soft tissue root coverage. They were only used with the purpose of providing a histologic description of gingival epithelium and connective tissue. Probing depth was performed immediately before tooth extractions; thus, portions of the junctional epithelium are not completely attached to the root surface (technical artifact) (100×) (a, b) and (200×) (c, d)
All epitheliums are underlined by a connective tissue layer of the lamina propria (“the connective tissue coat just beneath the epithelium and the basement membrane”) [1]. For the oral epithelium, the interface formed with the connective tissue is irregular and comprises “fingerlike projections” of connective tissue from the papillary layer (“connective papilla”) that extends into depressions on the undersurface of the epithelium (“epithelial crests”) [16]. These ridge-like projections of epithelium into the underlying stroma of connective tissue [1] (or rete pegs when seen in histological sections– Fig. 2.11) improve its attachment to the lamina propria [16]. The sulcular/crevicular epithelium also presents such “rete ridges” but in a diminished number, while at the junctional epithelium these are not present [17].
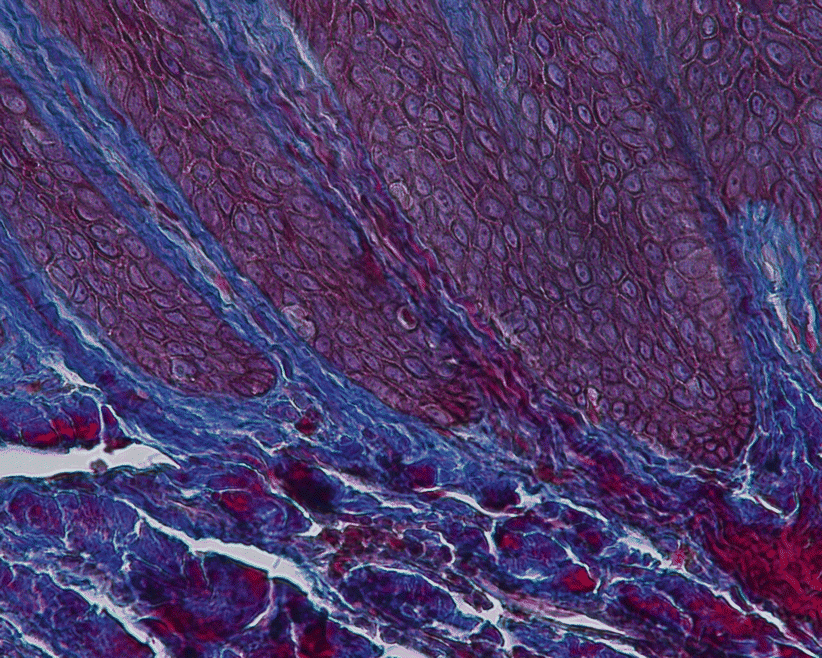

Fig. 2.11
Rete pegs (200×)
Regarding the components of the gingival connective tissue, it is formed by fibroblasts (65 % of all tissues) and other cells (i.e., mast cells, macrophages, undifferentiated mesenchymal cells, neutrophils, and plasma cells), fibers, blood vessels, and nerve fibers that are intended to act for cell nutrition, tissue healing, as well as mechanical (by the group of collagen fibers and the viscosity of the amorphous intercellular substance of the extracellular matrix) and phagocytic (via macrophages, neutrophils, antigen–antibody reactions, and activation of the complement system) mechanisms of defense [13, 15] Figs. 2.12 and 2.13.
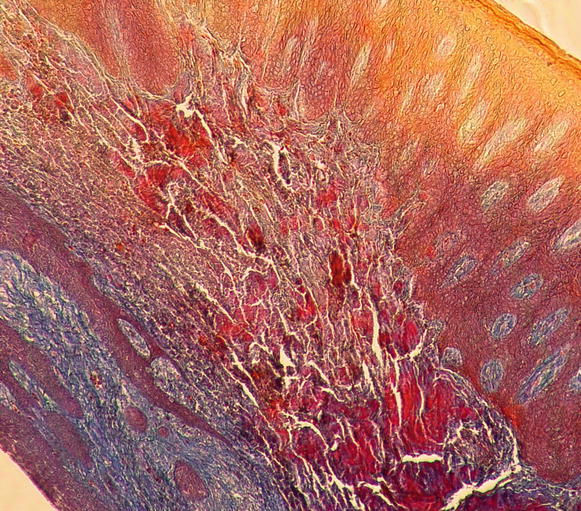
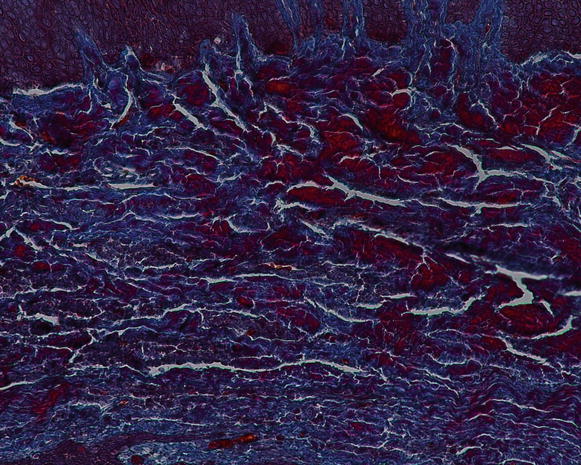

Fig. 2.12
Gingival connective tissue (100×)

Fig. 2.13
The gingival connective tissue (note the presence of the inflammatory cell within the tissue) (200×)
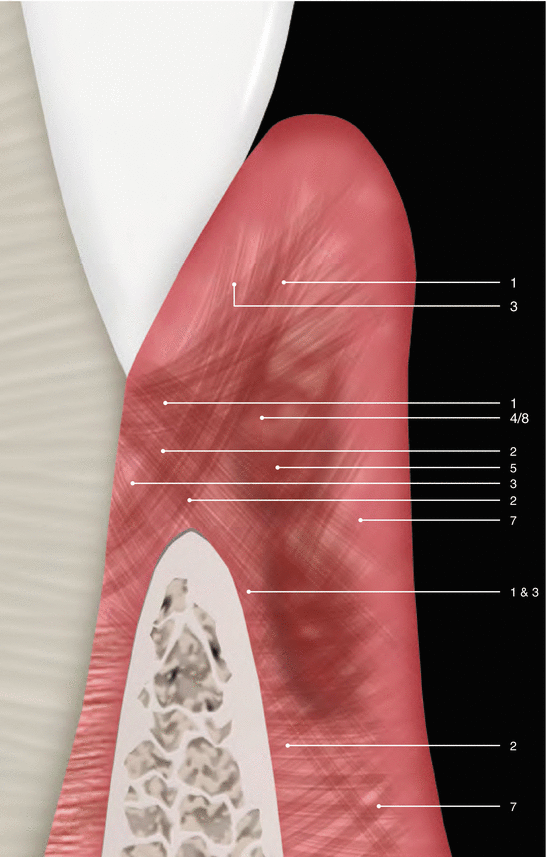
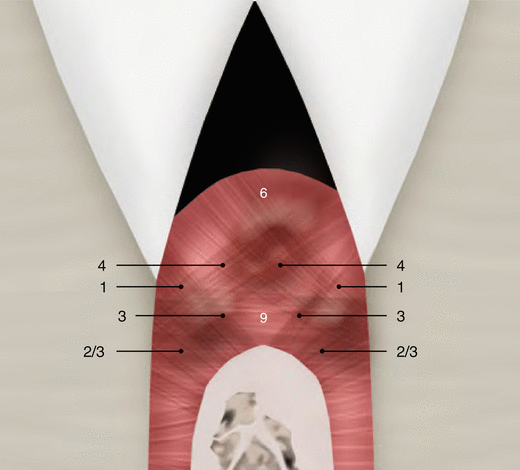
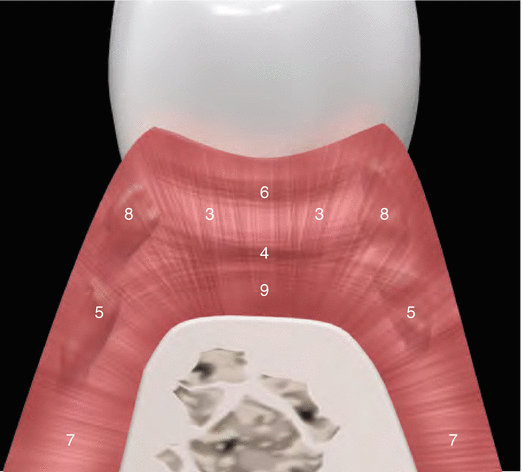
Although parts of the fibers are irregularly distributed through the connective tissue layer, most of them consisting of a dense network of collagen fiber bundles form an organized supra-alveolar fiber apparatus. Their function and location are illustrated in Figs. 2.14 and 2.15 (notes 1–9) and depicted as follows [1, 13, 15]:
Dentogingival fibers: Group of collagenous fibers that extend from the cervical cementum to the lamina propria of the free and attached gingiva (1).
Dentoperiosteal fibers: Group of fibers running from the cementum over the periosteum of the outer cortical plates of the alveolar process where they insert into the alveolar process or muscle in the vestibule of the floor of the mouth (2).
Alveolo-gingival fibers: Group of collagenous fibers that radiate from the bone of the alveolar crest into the lamina propria of the free and attached gingiva (3).
Circular/semicircular fibers: Group of collagenous fiber bundles within the gingiva that encircle the tooth in a ring-like fashion (4).
Intergingival and transgingival fibers: These groups of fibers are narrowly associated to the semicircular fibers. They ascend in the cementum and outspread through the intergingival fibers and interdental septum and eventually merge with the semicircular fibers of the adjacent tooth (5).
Interpapillary fibers: Group of collagenous fibers running between the gingival connective tissue of facial and lingual aspects of the posterior papilla (running in a buccolingual direction through the interdental tissue) (6).
Periosteo-gingival fibers: Group of collagenous fibers that extend from the periosteum of the outer cortical plates to the lamina propria of the gingiva (7).
Intercircular fibers: Group of fibers located both buccally and lingually and run in a mesiodistal manner to join circular fibers of adjacent teeth (8).
Transseptal fibers: Collagenous fibers that run interdentally from the cementum just apical to the base of the junctional epithelium of one tooth over the alveolar crest to insert into a comparable region of an adjacent tooth (9).
Additionally, other types of fiber bundles such as oxytalan (“fibers found in all connective tissue structures of the periodontium that appear to consist of thin, acid-resistant fibrils – their function is unknown”) [1], reticular (type III collagen fibers secreted by reticular/fibroblast cells that give support to the lamina propria of the gingiva), and elastic (around blood vessels) are present at the gingival connective tissue.
With respect to the neurovascular composition of the gingival connective tissue, the vascularization of the gingiva is primarily supplied by blood vessels located at the periodontal ligament and at the supraperiosteal portion of the lamina propria (i.e., supraperiosteal vessels) and by the intra- septal artery [13, 15]. These vessels/artery and capillary loops will originate from two vascular networks of arteriovenous anastomoses, the subepithelial and the dentogingival plexus (Fig. 2.16) [15]. Regarding the neural elements, these are its great majority surrounding/close to blood vessels and composed by myelinated fibers that play a sensory part [15].
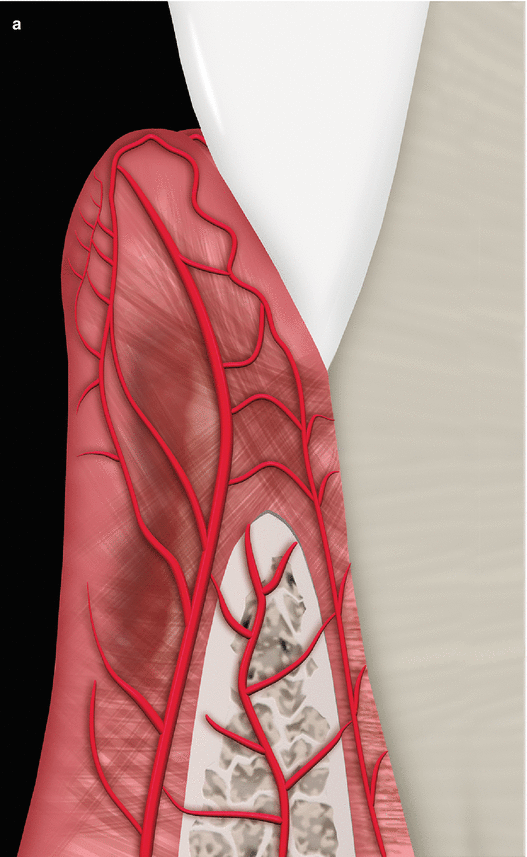
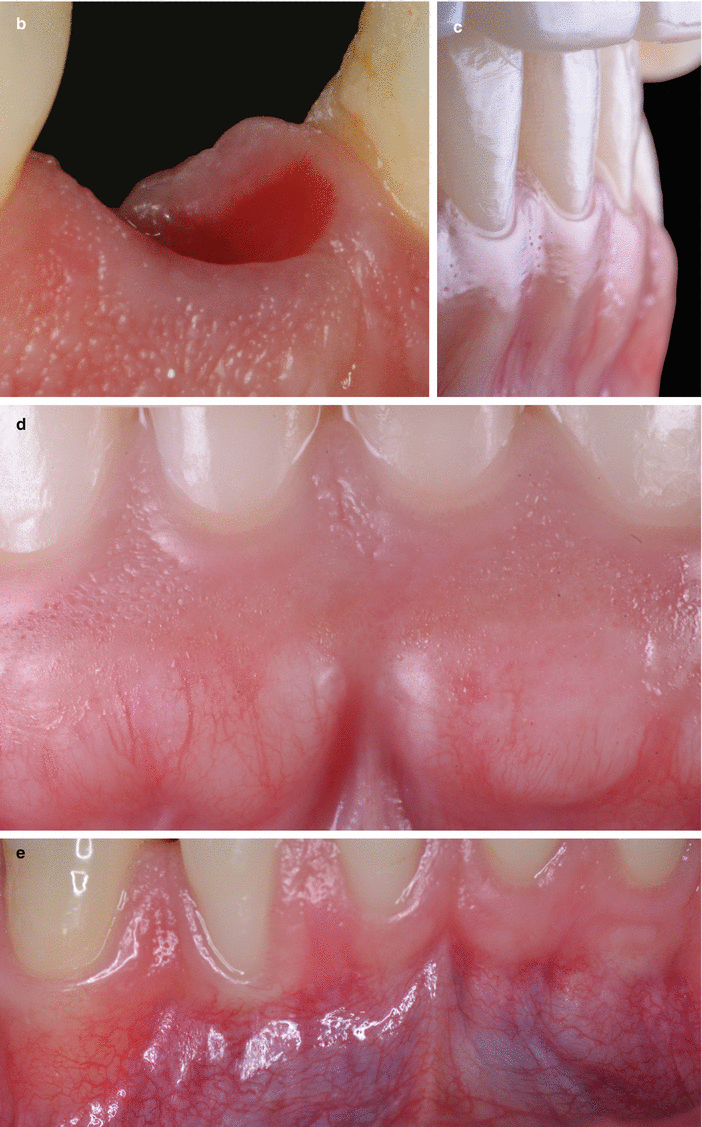


Fig. 2.16
Gingival vascularization
2.3 Anatomy of Supporting Periodontal Tissues: The Clinical Role of Cementum, Alveolar Bone, and Periodontal Ligament Conditions
The clinical role of the cementum, the alveolar bone, and the periodontal ligament conditions is briefly depicted below [1, 18]:
It is described as “the thin, calcified tissue of ectomesenchymal origin, similar to the alveolar, covering the roots of teeth which embedded collagen fibers attach the teeth to the alveolus” [1]. Its function is related to repair and attachment, as well as it is continuously deposited over a tooth’s life. It can be of acellular/primary (i.e., “that portion of the cementum that does not incorporate cells – it is formed during the root’s formation”) or cellular/secondary (i.e., “that portion of the cementum that contains cementocytes – it is formed during a tooth’s life in the apical third of the root as a response to occlusal forces”) origin (Fig. 2.17) [1, 18].
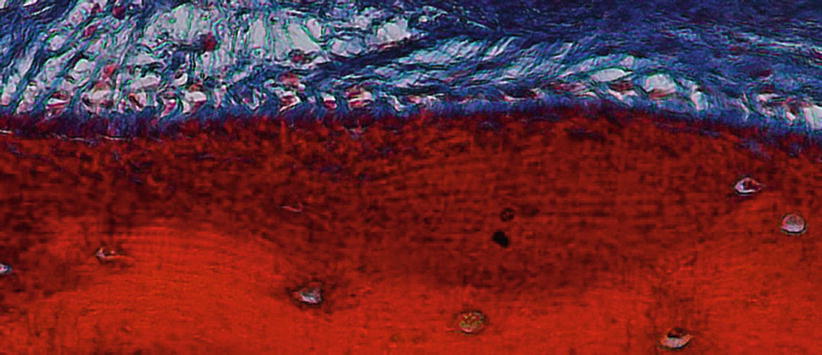
Fig. 2.17
Cementum and Sharpey’s fibers (200×)
Alveolar bone: “The hard form of connective tissue that constitutes the majority of the skeleton of most vertebrates. It consists of an organic component and an inorganic, or mineral, component. The organic matrix contains a framework of collagenous fibers and is impregnated with the mineral component, chiefly calcium phosphate and hydroxyapatite, that impart rigidity to bone. The alveolar process supports to alveoli, and consists of cortical bone, cancellous trabeculae, and the alveolar bone proper.” [1] It can be divided into alveolar bone proper (“compact bone that composes the alveolus (tooth socket), also known as the lamina dura or cribriform plate – the fibers of the periodontal ligament insert into it”) and basal bone (“the bone of the mandible and maxilla exclusive of the alveolar process”). Also, the alveolar bone may also be characterized by its structure as bundle (“a type of alveolar bone, so-called because of the “bundle” pattern caused by the continuation of the principal [Sharpey’s] fibers into it”), cancellous (“bone having a reticular, spongy, or lattice-like structure”), compact (“bone substance that is dense and hard”), or cortical bone (“the compact bone at the surface of any given bone”) [1]. The osseous morphology is dependent of the structures attached to the tooth, as well as to the structure and position of a tooth (Fig. 2.18) [18].
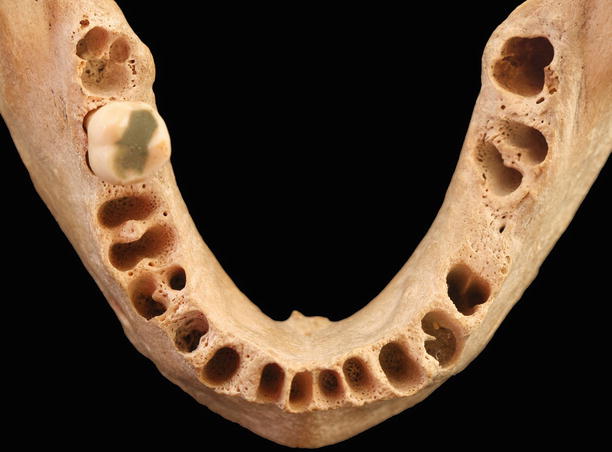
Fig. 2.18
Alveolar bone
Periodontal ligament: fibers that allow tooth attachment, and occlusal forces transmission to the alveolar bone (Sharpey’s fibers), protection to the neurovascular components, bone and cementum remodeling, and nutrition and proprioceptive sensitivity [18]. Among the fibers of the periodontal ligament, several types of cells may be found, such as fibroblasts, osteoblasts, osteoclasts, cementoblasts, cementoclasts, mesenchymal cells, epithelial cells, macrophages, and mast cells (Fig. 2.19) [18].
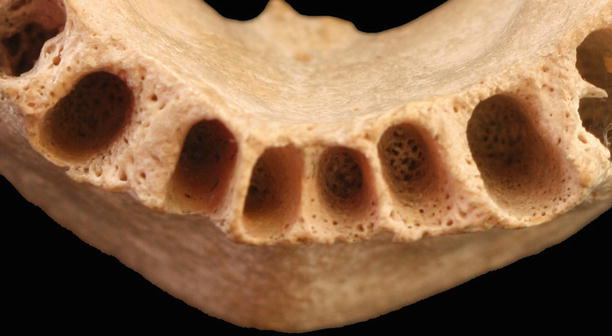
Fig. 2.19
Alveolar bone (closer view)
Together with the knowledge of the soft tissue anatomy, the characteristics of these tissues, mainly of the alveolar bone, play an important role on the development of soft tissue deformities even without the presence of dental biofilm. Anatomical features of the alveolar bone, such as bone fenestrations (i.e., window-like apertures or openings in the alveolar bone over the root of a tooth without comprising the marginal crestal bone) [1, 19–22] and dehiscence (i.e., areas in which the root is denuded of bone and portions of the root surface are covered only by soft tissue, and this area extends to through the marginal alveolar bone) [19–22], may be important for the development of periodontal disease and non-plaque-induced gingival lesions, as well as for the prognosis of a periodontal plastic surgery procedure (Fig. 2.20). For instance, approximately 10 % of the teeth may present fenestrations or dehiscence of the bone, but when accounting the presence of such defects “per subject,” this value increase up to 60 % [19]. Also, 12 % of maxillary central incisors may present lack of bone covering the root surface beneath the gingival tissues [20], and in the presence of malocclusions, the frequency of fenestrations and dehiscence may significantly increase to approximately 35 and 50 %, respectively, especially in the anterior region of the mandible (Figs. 2.21 and 2.22) [21, 22]. In addition, it should be noted that histological information indicates that the buccal bone wall is thinner than the lingual wall and both crests are located at a similar distance from the cementoenamel junction [23].
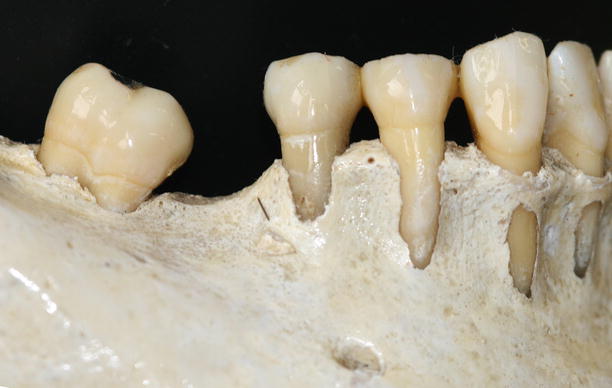
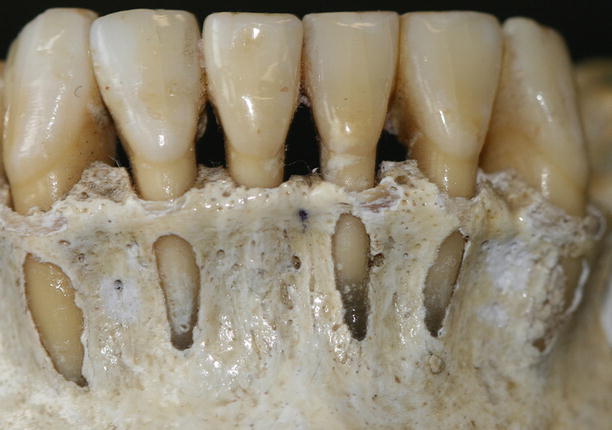
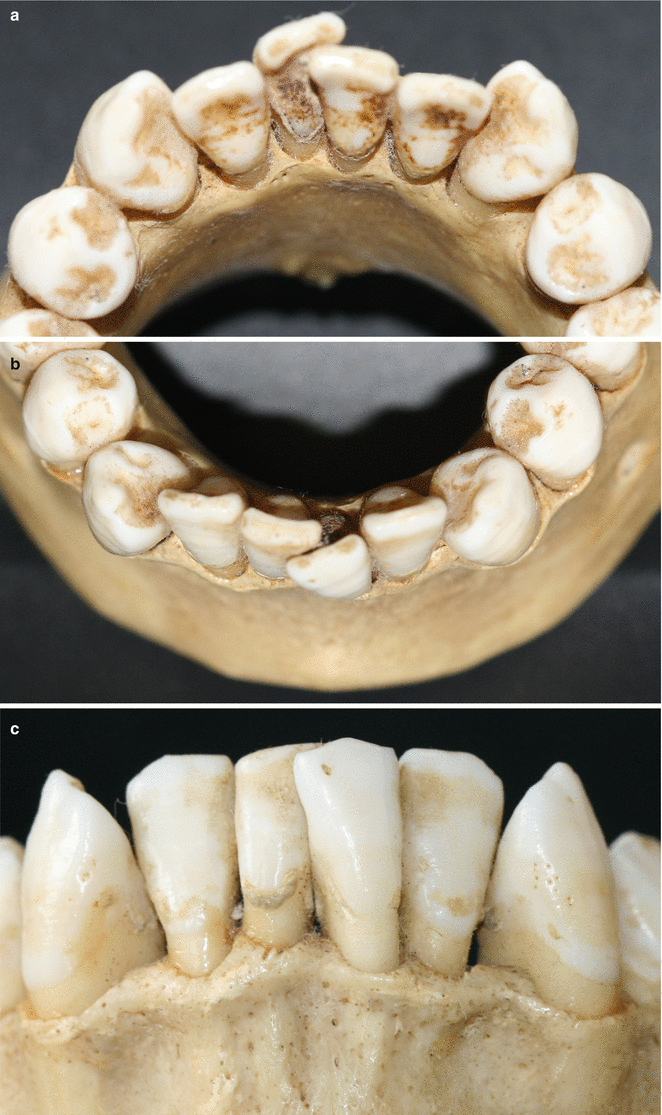

Fig. 2.20
Osseous dehiscence (premolars) and fenestrations (canine and lateral incisor) at the mandibular arch

Fig. 2.21
Osseous fenestrations (canines and incisors)

Fig. 2.22
(a–c) Anatomic conditions of the alveolar bone around crowded teeth
2.4 Additional Anatomic Conditions to Be Considered During the Decision-Making Process for Gingival Surgery: The Palatal Vault, the Periodontal Biotype, the Keratinized Tissue Band Around Natural Teeth, and the Biological Width
The palatal vault may play an important role during the decision-making process due to its inherent anatomical characteristics, in terms of potential damages and complications (e.g., small bleeding up to hemorrhage) that may occur to the greater palatine artery and their major branches [24, 25]. Known as the main artery supplying blood to the masticatory mucosa of the palatal vault, the greater palatine artery emerges on to the inferior surface of the hard palate through the greater palatine foramen and follows anteriorly close to the alveolar ridge up to the incisive foramen, where it leaves the palate (Fig. 2.23) [24–27]. Also, the greater palatine nerve accompanies this artery, and it is related to anesthetic procedures of the anterior teeth and palatal mucosa [24–28].
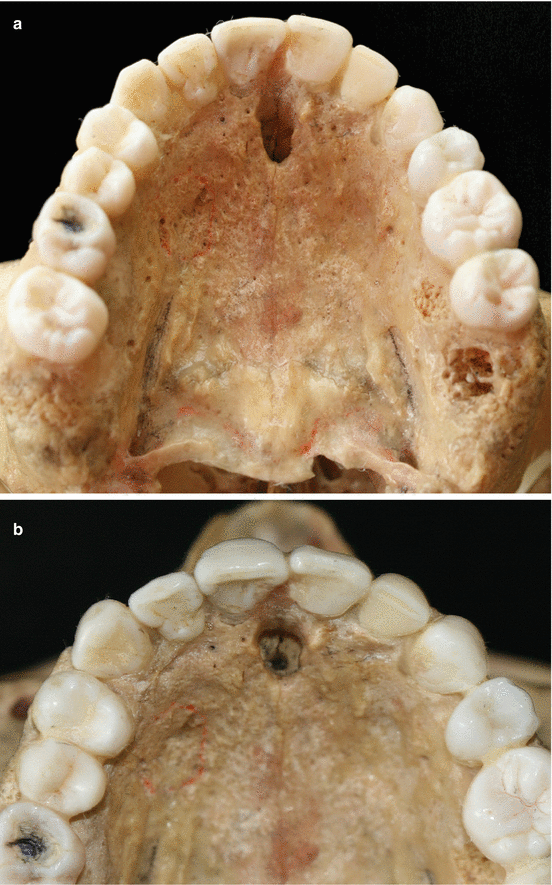
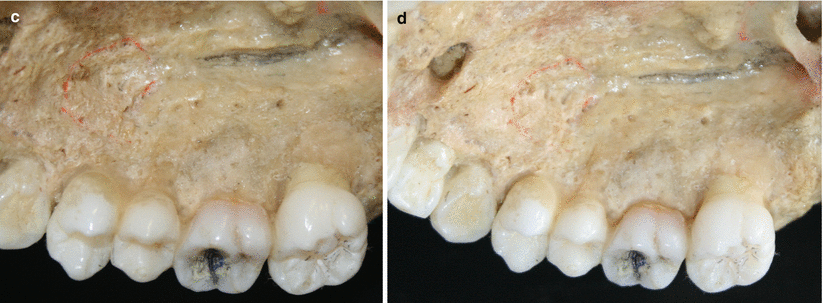
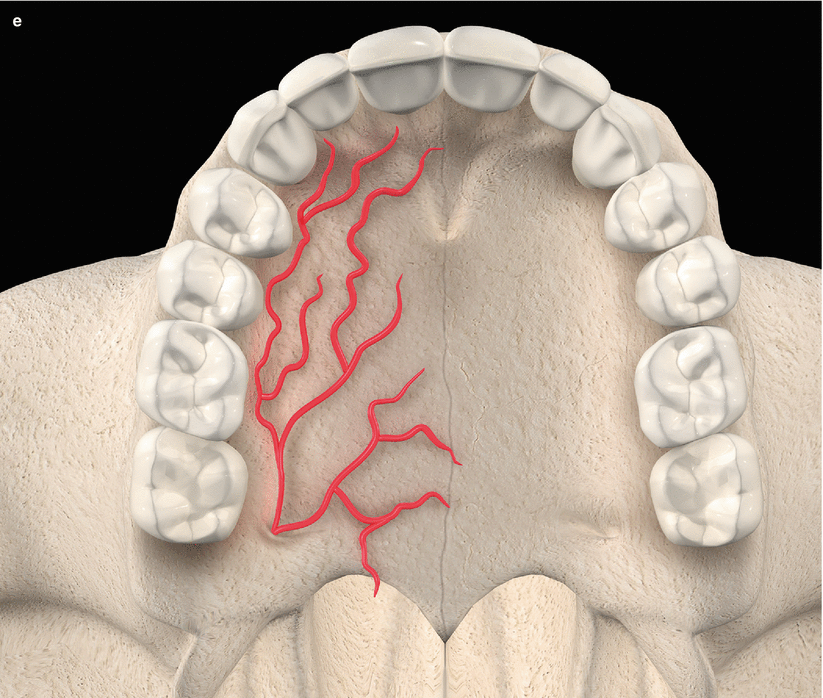



Fig. 2.23
Palatal vault – incisive foramen (a, b), greater palatine foramen (c, d), and schematics of the greater palatine artery (e)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree