Chapter 17 Peptides and Proteins
TERMINOLOGY AND DEFINITIONS
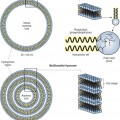
Peptides are short chains of amino acid sequences that make up larger proteins. There are three main categories of peptides currently being used in cosmeceutical products (Box 17.1). This increase in peptide technology has arisen because of the technology to synthesize fragments that mimic peptide sequences in collagen and elastin with the ability to stimulate production of new collagen and elastin. Other peptides are currently available that function primarily as carriers of cofactors for important enzymatic steps in collagen production. Peptide fragments also exist that are able to modulate neurotransmitter release. Since some wrinkling of the skin is caused by collagen breakdown, while other wrinkles are caused by hyperkinetic facial muscle movement, peptides that have actions to inhibit or reverse these actions could have clinical antiaging cosmeceutical benefits.
INDICATIONS AND BIOLOGIC ACTIVITY
• Signal peptides
It is well recognized that the ability to stimulate proteins of the extracellular matrix, including collagen and elastin, or to diminish the activity of collagenase, or both, could result in clinical improvement of the wrinkles and lines that are seen in both photoaged and naturally aged skin. Thus, direct stimulation of collagen-producing human fibroblasts and/or downregulation of fibroblast collagenase production are the mechanisms by which a cosmeceutical may clinically improve lines and wrinkles. Bioactive peptides were originally developed as part of wound-healing research on the growth and stimulation of human skin fibroblasts. These same peptides are now being studied for their ability to act as growth factors via activation of protein kinase C, a key enzyme for cell growth and migration. Wound studies of human keratinocyte cultures show a stimulatory effect from topical application of neuropeptides, such as gastrin-releasing peptide. Certain amino acid chains in specific lengths and sequences have been found to stimulate human skin dermal fibroblast growth in vitro. One study of elastin-derived peptides showed that valine-glycine-valine-alanine-proline-glycine (VGVAPG) significantly stimulated human skin fibroblast production, probably mediated through a binding of the peptide to a plasmalemmal receptor of human skin fibroblasts. This same hexapeptide sequence has been found to stimulate human dermal skin fibroblasts, downregulate elastin expression, and to be chemotactic for fibroblasts. Other studies have shown the peptide sequence tyrosine-tyrosine-arginine-alanine-aspartame-aspartame-alanine (YYRADDA) to inhibit procollagen C-proteinase, which cleaves C propeptide from type I procollagen. This could result in decreased collagen breakdown. A specific amino acid sequence, lysine-threonine-threonine-lysine-serine (KTTKS), found on type I procollagen, has been found to stimulate feedback regulation of new collagen synthesis, resulting in an increased production of extracellular matrix proteins. A number of these signal peptides have made the transition from research to practical application.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree