Fig. 3.1
(a, b) Clinical appearance of pemphigus herpetiformis with annular, urticated, erythematous plaques over the trunk, arms and legs (From Laws et al. [49]. Reprinted with permission from John Wiley and Sons)
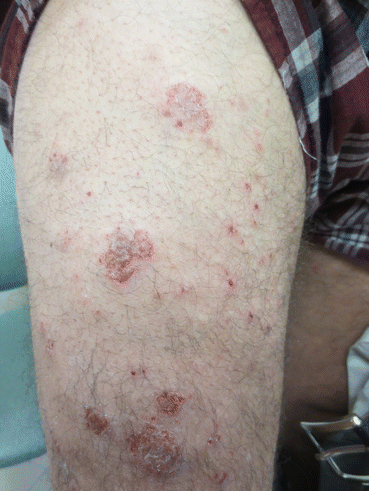
Fig. 3.2
Clinical appearance of pemphigus herpetiformis with vesiculobullous erythematous plaques (From Laws et al. [49]. Reprinted with permission from John Wiley and Sons)
Histopathology
The histopathological features of PH are variable over time and multiple biopsies may be required, particularly when the diagnosis is not considered and immunofluorescence studies are not performed. Typical histological findings include an eosinophilic spongiosis and intraepithelial blisters (subcorneal or suprabasal) that may include eosinophils or neutrophils [9]. Studies report eosinophilic spongiosis in 20 %, neutrophilic spongiosis in 20 % and a mixed neutrophilic and eosinophilic spongiosis in 60 % (Figs. 3.3 and 3.4) [9]. Acantholysis may be absent or only detectable after multiple biopsies or a protracted disease course.
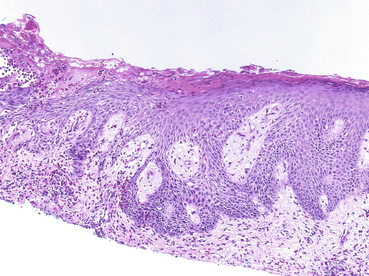
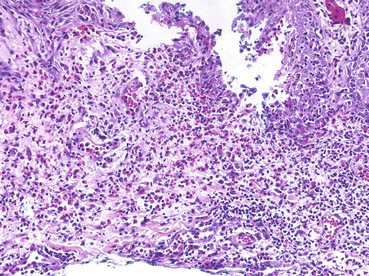

Fig. 3.3
Skin biopsy (×100, HE) demonstrating eosinophilic and neutrophilic spongiosis with focal suprabasal acantholysis (From Laws et al. [49]. Reprinted with permission from John Wiley and Sons)

Fig. 3.4
Skin biopsy (×200, HE) demonstrating eosinophilic and neutrophilic spongiosis with focal suprabasal acantholysis (From Laws et al. [49]. Reprinted with permission from John Wiley and Sons)
Immunopathology
Direct Immunofluorescence (DIF)
DIF findings for PH typically overlap with both PF and/or PV. Intercellular deposits of immunoglobulin (Ig) G and/or complement (C3) is typical and may be suprabasal or subcorneal [9]. Increasing numbers of case reports have been published detailing unusual additional findings in patients with PH including the presence of IgA antibodies within the intercellular space and IgG binding to the basement membrane [10]. The significance of this remains to be determined and is discussed in more detail below.
Indirect Immunofluorescence
PF is characteristically associated with antibodies to Desmoglein (Dsg)1 while PV is associated with antibodies to Dsg3. The majority of patients with PH reported to date have antibodies to Dsg1 or Dsg3 [11]. Ishii et al. have reported on sera of 20 patients with PH demonstrating reactivity to Dsg1 and Dsg3 in 80 % (n = 16/20) and 20 % (n = 4/20) respectively [11]. Of note in this study no patients demonstrated reactivity to both Dsg1 and Dsg3. A smaller study of 7 patients reported in Brazil (endemic for PF) reported antibodies only to Dsg1 [12]. Recent studies have suggested that PH may have a broader autoantibody profile and that in some patients antibodies may be directed at desmocollin glycoproteins [13, 14].
Disease Association
Due to the small number of cases reported in the literature it is unclear if there is any disease association with PH. Case reports detailing an association with malignancy include lung cancer [15–17], esophageal cancer [18], and prostate cancer [19]. Of note PNP is generally an aggressive, treatment resistant form of pemphigus with striking mucosal involvement. If an association with PH and malignancy was established, the diagnostic criteria for PNP may need to be adapted to ensure clear distinction between pemphigus subtypes.
Other diseases reported in association with PH include psoriasis [20, 21], systemic lupus erythematosus [22], autoimmune haemolytic anaemia [23], and Human Immunodeficiency Virus [24]. In one patient with psoriasis it would seem that PH may have been precipitated by ultraviolet light therapy prescribed as treatment for psoriasis [21].
Diagnosis
Differential Diagnosis of Pemphigus Herpetiformis
Pemphigus vulgaris
Pemphigus foliaceus
Bullous pemphigoid
Linear IgA Bullous Dermatosis
IgA pemphigus
IgG/IgA pemphigus
Atopic dermatitis
Allergic contact dermatitis
Drug-induced eruption
Dermatitis herpetiformis
The differential diagnosis of PH is broad and frequently the diagnosis is overlooked. The differential diagnosis includes PV, PF, bullous pemphigoid, linear IgA bullous dermatosis, dermatitis, drug rashes, IgG/IgA pemphigus and allergic contact dermatitis. Table 3.1 provides a summary of key findings of some of these diseases. IgG/IgA pemphigus is a newly described disease that requires further investigation. Research to date would indicate that this entity overlaps with PH but may be distinguished from PH due to the presence of IgG and IgA antibodies to Dsg1 [27].
Table 3.1
Differential diagnosis of pemphigus herpetiformis and distinguishing features
Clinical presentation | Oral involvement | Histopathology | IMF | Autoantigen | Peripheral blood eosinophilia | |
|---|---|---|---|---|---|---|
Pemphigus herpetiformis | Pruritic urticated erythema, vesiculobullous | Rarely | Eosinophilic spongiosis, intraepidermal neutrophils, acantholysis (may be absent), intraepithelial blisters, | IgG | Dsg1, Dsg3 | ++ |
Pemphigus vulgaris | Flaccid blisters, erosions | Yes, may be dominant feature of disease | Suprabasal acantholysis | IgG | Dsg3 ± Dsg1 | + |
Pemphigus foliaceus | Superficial blisters, crusted erosions (may be seborrheic in distribution) | No | Subcorneal acantholysis | IgG | Dsg1 | + |
Linear IgA Bullous dermatosis | Vesiculobullous (“string of pearls” pattern) | No | Subepidermal blister with predominant neutrophilic infiltrate | Linear IgA BMZ | LAD-1, BP180 | − |
Bullous pemphigoid | Pruritic urticated erythema, tense blisters | No | Subepidermal blister with eosinophilic infiltrate | IgG BMZ | BP180, BP230 | ++ |
The first description of PH by Jablonska et al. in 1975 proposed diagnostic criteria including clinical features of dermatitis herpetiformis and direct immunofluorescence findings of pemphigus [1]. Since then diagnostic criteria have been developed to include urticated erythema and/or vesiculobullous lesions in the context of pruritus. Current proposed diagnostic criteria are summarized below. While this is typical it should be emphasized that these criteria are not definitive and reports of PH in the absence of Dsg1 or Dsg3 have been reported [28].
Pemphigus Herpetiformis: Diagnostic Criteria
Clinical
Pruritus
Urticated erythema (± blister or erosion)
Histopathology
Eosinophilic/neutrophilic spongiosis
Variable acantholysis
Immunofluorescence
Epidermal intercellular deposits of IgG
NB. These criteria are not exclusive to pemphigus herpetiformis and should be interpreted in light of clinical scenario
Pathogenesis
Pemphigus is an autoimmune disease of epidermal cell adhesion with antibodies directed at components of the desmosome. In the majority of patients with pemphigus antibodies to desmoglein, a protein of the cadherin superfamily drives this. Cadherins are broadly categorized as classical cadherins or desomosomal cadherins. The desomosomal cadherins include desmogleins and desmocollins.
Epidermal cell adhesion is maintained through adherin and desomosomal junctions. Adherin junctions typically form weak associations while desmosomes provide structural integrity. Transmembrane desmogleins and desmocollins form the extracellular component of the desmosomal plaque and interact with intracellular desmoplakins, plakoglobins and plakophilins (Fig. 3.5). These latter proteins provide a point of binding for intracellular keratin.


Fig. 3.5
Diagrammatic representation of desmosome. Dsc desmocollin, Dsg desmoglein, PG plakoglobin, PP plakophilin, DP desmoplakin
There are four isoforms of desmoglein (Dsg1-4) and three isoforms of desmocollins (Dsc1-3). Dsg1 and Dsg3 are essential to epidermal function. Dsg1 is found predominantly within the upper epidermis (stratum granulosum) while Dsg3 is located within the lower levels of the epidermis (stratum basale and spinosum). As discussed in previous chapters PV is typically caused by antibodies directed at Dsg1 and Dsg3. Mucosal desmosomes are predominantly mediated by Dsg3 and antibodies directed at Dsg3 are therefore key in the development of mucosal disease. It is therefore interesting to note that despite Dsg3 antibodies in PH mucosal lesions are rare. Patients with PF develop antibodies to Dsg1, which are largely absent from the mouth, and consequently experience skin limited disease.
Recent studies have provided evidence that antibodies other than Dsg1 or Dsg3 may induce PH. A case report by Tateishi et al. describe a case of PH with antibodies to Dsc1 only [29]. The role of Dsc in pemphigus disease is increasingly being examined in greater detail and appears to be significant. Spindler et al. have reported that desmocollins are important in the pathology of pemphigus through interactions with Dsg1 but not Dsg3 [30].
Classical pemphigus disease (both PF and PV) results in acantholysis, blister formation and clinical disease. Despite the presence of Dsg 1 or Dsg3 in PH the relative lack of acantholysis and blistering is notable. The underlying mechanism behind acantholysis and blistering remains to be fully explained but several mechanisms have been proposed. These include:
1.
Steric hindrance – Antibodies directed at anchoring proteins disrupt cell adhesion and prevent attachment [31]. Antibodies in PH may be different and therefore not induce acantholysis to the same extent.
2.
3.
Acantholysis would appear to be dependent on pemphigus gamma-globulins and it has been suggested that this protein may not be elevated in PH [34].
4.
Epitope recognition – Specific antigenic epitopes may be influential in determining disease expression. Epitope recognition perhaps offers the best explanation for differential expression of acantholysis in patients with apparently the same antibody profile [35].
It is increasingly clear that pemphigus subtypes may change over time in some patients. This occurs to such an extent that PF may develop in to PV or vice versa [36, 37]. This is also supported by Maciejowska et al. who reported 33 % (n = 5/15) of patients with PH progressed to classical PF later in the disease course [7]. It has been proposed that this occurs through epitope spreading [38].
The mechanism of epitope spreading remains unclear. One explanation would be that the inflammatory milieu in pemphigus disease enhances further epitope recognition and cellular damage results in exposure of previously unexposed epitopes. With an enhanced spectrum of antibodies recognising multiple epitopes clinical expression of disease may change. This is the source of significant research in the literature with intramolecular and intermolecular epitope spread hypothesized [39, 40]. The theory of intramolecular epitope spreading is supported by Lebeau et al. who report a patient with PH who initially developed Dsg3 antibodies to ectodomain 1 of the protein which changed over the duration of disease to ectodomain four predominant expression [41]. This change in antibody profile was accompanied by development of mucosal disease. It would appear that PH has a broader epitope distribution than PV and perhaps provides evidence that PH may represent an undifferentiated pemphigus phenotype which in some patients later progress to PV or PF [38].
The complexity of immunobullous disease is further evident in a recent report of PH and mucous membrane pemphigoid in the same patient [13]. Direct immunofluorescence (DIF) demonstrated strong IgG and weak IgA to keratinocyte surface with C3 at the basement membrane zone. Further studies demonstrated IgG antibodies to Dsc-1 and IgG antibodies to BP180 and laminin 332. The authors hypothesized that intermolecular epitope spreading between Dsc1 and BMZ antigens may explain the dual pathology evident in their patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






