(1)
RZANY & HUND Privatpraxis für Dermatologie und Ästhetische Medizin, Kurfüstendamm, Berlin, Germany
1.1 Introduction
1.3.1 Collagen
1.3.2 Hyaluronic Acid
1.3.4 Alginates
1.3.5 Poly-l-lactic Acid
1.3.6 Calcium Hydroxylapatite
1.4.1 Silicone
1.4.2 Polyacrylamide
1.4.3 Polyalkylimide
Abstract
In contrast to the USA, in most countries in Europe and South America, a great variety of injectable fillers are available. Therefore, not only for novices but also for experienced users it can sometimes be quite difficult to decide which filler to use. This chapter will give a brief overview on some of the most commonly used present and past injectable fillers. The selection of products reflects the interest of the authors and might appear arbitrary to someone familiar with other fillers.
1.1 Introduction
In contrast to the USA, in most countries in Europe and South America, a great variety of injectable fillers are available. Therefore, not only for novices but also for experienced users it can sometimes be quite difficult to decide which filler to use. This chapter will give a brief overview on some of the most commonly used present and past injectable fillers. The selection of products reflects the interest of the authors and might appear arbitrary to someone familiar with other fillers.
FAQs
Why should one show interest with fillers which are not on the market anymore?
Even when fillers are not present on the market anymore, they may be important for two reasons: (1) they may be marketed again, and (2) permanent fillers will be always present as they will stay with the patient until the end.
1.2 Classification of Fillers
Basically there is no uniformly accepted classification of fillers. Injectable fillers may be grouped according to (1) the degree of degradability and (2) the quality of the clinical data behind them.
1.2.1 Classification by Biodegradability
Fillers can be grouped as biodegradable and nonbiodegradable (permanent) products. There are also fillers where biodegradable as well as nonbiodegradable materials are combined.
1.2.2 Classification by the Quality of Clinical Data
As the rules for marketing of fillers are quite relaxed in Europe, e.g., a clinical trial is not required, fillers can be grouped in those with and without clinical data. Those with clinical data can be grouped in those with good and less good clinical data.
What means good clinical data? Basically a randomized controlled clinical trial with a sufficient number of patients included (e.g., for a two-arm trial you want at least 50 patients). Based on such a trial simple but important questions as grade of correction that can be achieved, durability of the correction (e.g., efficacy), impact on quality of life, and safety (proportion of patients with swelling, etc.) can be answered.
1.3 Biodegradable Fillers
Biodegradable fillers are defined as having a limited life span usually ranging from a couple to several months, or even to a couple of years. They consist of purified dermal components from human, animal, or bacterial sources and can be divided into the following categories: xenografts (donor and recipient are from different species), autografts (donor and recipient are from the same individual), homografts (donor and recipient are from the same species), and synthetic materials (Table 1.1). Please note that in the last couple of years some of the most well-known biodegradable fillers were removed from the market.
Table 1.1
Overview on biodegradable fillers
Material | Origin | Productsa |
|---|---|---|
Temporary injectable fillers | ||
Alginate | Nonanimal, algae | Novabelb |
Collagen | Bovine | Zydermb, Zyplastb |
Porcine | Evolenceb | |
Human (cadaver derived) | Cymetrab | |
Human (self-derived) | Isolagenb | |
Human (cultivated) | Cosmodermb, Cosmoplastb | |
Hyaluronic acid | Avian | Hylaformb |
Nonanimal | Beloteroc, Emervelc, Juvédermc, Restylanec, Teosyalc, Juvéderm Volumac | |
Hyaluronic acid + dextran | Nonanimal | Reviderm |
Poly-l-lactic acid (PLLA) | Nonanimal | Sculptra (former New Fill) |
Calcium hydroxylapatite | Nonanimal | Radiesse |
1.3.1 Collagen
Collagens from various sources and with specific characteristics exist or better used to exist as most of the fillers discussed are not on the market anymore.
1.3.1.1 Collagen of Bovine Origin
Prior to the introduction of the hyaluronic acids, collagen was the most widely used filler and was considered the gold standard with which other dermal fillers were compared. However, the role of bovine collagen is declining. In the USA and in Europe, they are not available on the markets anymore. Nevertheless, bovine collagen still might be available in other parts of the world, and therefore we will give a brief overview on that substance. The classical bovine enzyme-digested collagen (95 % type I, 5 % type III) was available in several preparations, which were distinguished by the collagen content and the addition of glutaraldehyde for stabilization (Homicz and Watson 2004).
Depending on the collagen content and the degree of cross-linking, different products were designed for different levels of the dermis. For example, Zyderm 1 and Zyderm 2, which were built on noncross-linked collagen, were supposed to be injected into the superficial dermis. Zyplast, a cross-linked form, was supposed to be injected more deeply into the dermis. All of these products were easy to inject. In contrast to other products, overcorrection was recommended for Zyderm 1 and Zyderm 2.
Zyderm was cleared for marketing in 1981 by the Food and Drug Administration (FDA) after reviewing clinical data based on a large case series of 9,427 tested and 5,109 treated patients (Cooperman et al. 1985; Matti and Nicolle 1990). In addition to this case series, which focused mainly on safety issues, a further clinical trial showed that it was effective for at least several months (Cooperman et al. 1985; Matti and Nicolle 1990).
As collagen may elicit quite often hypersensitivity reactions, pretesting was mandatory. Pretesting consisted of an intradermal injection of Zyderm 1 collagen into the volar aspect of the forearm. A minimum of one skin test which was valuated after 28 days was required. The incidence of adverse reactions to collagen pretesting (here Zyderm I) was approximately 3 %. Of all test site reactions, 50 % occurred within the first 24 h. An additional 1.3 % of patients experienced adverse reactions despite a negative pretest. The observed reactions ranged from localized swelling to induration, erythema, and pruritus (Cooperman et al. 1985).
Key Points
So far, bovine collagens are not available anymore in Europe and the USA. There might be two reasons for that: (1) the need of prior skin testing and (2) the decreased durability compared to the hyaluronic acid preparations.
As bovine collagen was the comparator in the initial non-collagen filler trials, the evidence behind this group of products is good.
1.3.1.2 Collagen of Porcine Origin
Before Evolence there were only a very few reports on porcine collagen-based fillers in the literature (Saray 2003). Evolence, a novel porcine collagen filler, was introduced into the European market in 2004 and withdrawn from the markets in 2009. In contrast to other collagens, this product was cross-linked by glycation using d-ribose as the cross-linking agent. Unlike bovine collagen, no skin testing was necessary.
This porcine collagen was available in two preparations: Evolence and Evolence Breeze. Evolence Breeze was indicated for more superficial dermal injections and lip augmentation.
Being a new filler, efficacy was supported by a couple of good clinical trials (Monstrey et al. 2007; Narins et al. 2007, 2008). Furthermore, some smaller case series were reported focusing on specific areas as nonsurgical rhinoplasty (Cassuto 2009), tear trough correction (Goldberg 2009), cheek augmentation (Sadick and Palmisano 2009), and lip augmentation (de Boulle et al. 2009; Landau 2009).
The risk of hypersensitivity reactions for porcine collagen was much lower compared to bovine collagen. In an intradermal skin test study, no hypersensitivity reactions could be detected in a total of 519 subjects (Shoshani et al. 2007). Therefore, no skin testing was recommended. There are, however, a few reports on foreign body reactions inducing an abscess-like reaction (Braun and Braun 2008). As several thousands of patients had been treated before the withdrawal, this risk seemed to be comparable to other biodegradable fillers.
Porcine collagen was not as easy to inject compared to bovine collagen. By mixing lidocaine (0.2 ml) to the syringe, the injectability as well as the injection comfort (less pain!) could be increased. As for Like bovine collagen, the filler had a yellowish color which could be visible beneath the mucosal surfaces when the filler was injected too superficially. For this filler, a guideline is available (Rzany et al. 2010).
Key Points
Evolence was a filler which was supported by good clinical data.
With the withdrawal of the Evolence products, no porcine filler is at the moment available in Europe and the USA.
Nevertheless, it cannot be ruled out that this or a comparable product will be reintroduced to the market again.
1.3.1.3 Collagen of Human Origin
Collagen of human origin can be of allogenous or autologous nature.
1.3.1.3.1 Collagen of Allogenous Nature (From Cadaver)
In addition to bovine or porcine sources, collagen can be derived from human cadavers. Data is available for two products: Dermalogen and Cymetra. Both products derive from pooled human cadaverous tissue from accredited tissue banks. Overcorrection is recommended by the manufacturer. Here again the available data on the efficacy and safety of the product are limited. Cymetra was tested against Zyplast in a small randomized controlled trial. A total of 47 patients were treated: 20 received Cymetra and 27 received Zyplast. Various photometric outcome measures were used in this study, which favored the new product over Zyplast (Sclafani et al. 2002a, b).
1.3.1.3.2 Collagen of Allogenous Nature (From Culture)
Later-generation noncadaverous collagen products are Cosmoderm and Cosmoplast (Baumann 2004). They were made from natural human collagen grown under controlled laboratory conditions. There was no need for a pretreatment skin test for these sterile devices, which were composed of highly purified human-based collagen that is dispersed in phosphate-buffered physiological saline containing 0.3 % lidocaine. Cosmoderm was a noncross-linked formulation that was used in the treatment of superficial lines, whereas Cosmoplast was cross-linked and was used primarily in the treatment of more pronounced wrinkles. A few clinical trials are available using Cosmoderm as a comparator. Based on these trials, the durability seems to be less as for other products (Man et al. 2008; Smith et al. 2007).
1.3.1.3.3 Collagen of Autologous Nature
The commercial preparation Autologon consists of dermal extracellular matrix, primarily collagen (types I, III, and VI), that has been harvested from the patient’s own skin. It requires the excision of the patient’s skin and is therefore mostly suitable for those undergoing surgical procedures. Here again, overcorrection is recommended by the manufacturer. The available data on the efficacy and safety of the product are limited (Sclafani et al. 2000).
Concerning the safety as said before, the number of studies for the above products is limited. Pretesting might reveal adverse self-limited local reactions (Moody and Sengelmann 2000). Adverse reactions after pretesting appeared only as mild, nontender erythema. Acute or severe reactions like allergic ulcerations or chronical granulomatous reactions were not reported in a nonsystematic review (Fagien 2000). Case reports describe acute choroidal infarction following the subcutaneous injection of allogenous collagen in the forehead region (Apte et al. 2003).
Key Points
This overview is merely for academic reasons. The products to our knowledge are either not widely used or not available anymore.
The quality of clinical data behind these products varied.
1.3.2 Hyaluronic Acid
After the bovine collagens, the emergence of different hyaluronic acid preparations revolutionized the injectable filler market for three main reasons: (1) no need for skin test, (2) better durability compared to the other available biodegradable fillers, and (3) the availability of antidote (hyaluronidase).
Hyaluronic acid, which belongs to the family of glycosaminoglycans, consists of repeated disaccharide units. The hydrophilic properties of hyaluronic acid attract water into the extracellular matrix and therefore increase the skin turgor. Hyaluronic acid is gradually degraded. In order to increase the durability of the various hyaluronic acid preparations, stabilization is usually obtained by cross-linking mostly with 1.4-butanediol diglycidyl ether (BDDE).
Hyaluronic acids can be derived from avian or bacterial sources; each product has its own, specific characteristics.
Key Points
HA can be derived from different sources. Most HAs derive from bacterial origin.
1.3.2.1 Hyaluronic Acid of Avian Origin
Cross-linked hyaluronic acid of avian origin became the first non-collagen filler to be widely used. However, it is not available anymore. The Hylaform product family was based on hyaluronic acid derived from processed rooster combs. The Hylaform product family, with an average content of hyaluronic acid of 5.5 mg/ml, was easy to inject due to its good rheological properties and is less palpable than some products of bacterial origin (Manna et al. 1999).
In 2003, data from a clinical trial comparing Hylaform with Zyplast for the treatment of nasolabial folds was presented to the FDA. A total of 480 patients were included in this study which, to our knowledge, was never published. Based on the data that are available from the FDA, no difference between the products could be established. After 12 weeks the mean (±standard deviation) wrinkle severity score, which ranged from 0 to 5, was 3.3 ± 1.11 for Hylaform and 2.2 ± 1.12 for Zyplast (http://www.fda.gov/).
Key Points
Hylaform is not available anymore.
The product was based on good clinical data.
1.3.2.2 Hyaluronic Acid of Bacterial Origin
HA preparations of bacterial origin dominate the market. They are quite a heterogeneous group and sometimes quite confusing to differentiate as each company seems to have its specific wording to make it look more different from another one.
Basically they can be differentiated in products with good and in products with not-so-good or even nil clinical data.
1.3.2.2.1 Products with Good Clinical Data
The Q-Med Restylane Family
The Restylane family is the hyaluronic acid family with the best evidence behind it. The reason for that is as said before that after the bovine collagens it became the gold standard for comparative trials in the USA.
The first trial published was a randomized controlled clinical trial conducted to compare the efficacy and safety of Restylane and Zyplast. A total of 137 patients were included in the intention-to-treat analysis. After 6 months the authors concluded that Restylane was superior to Zyplast (based on the assessment of the Winkle Severity Rating Scale). The superiority of Restylane (i.e., where the investigator felt that Restylane was more effective) was observed in 56.9 % of their patients, compared to 9.5 % patients in whom the investigator felt that Zyplast was superior (p < 0.0001). Those patients in whom there was no difference between these products (33.6 %) were not included in the simple univariate statistics (Narins et al. 2003).
The Restylane family is sometimes described as biphasic HAs. What does biphasic mean? Basically it is a cross-linked HA product that is formed in particles which are enclosed by noncross-linked HA. As noncross-linked HA is degraded easily, the initial achieved correction might not be as good as this has been shown in the Emervel Deep and Teosyal Deep trials where the products were compared to Restylane Perlane (Nast et al. 2011; Rzany et al. 2011). The particle size determines the indication. The HA products with smaller particles are intended for more superficial use.
The Q-Med Emervel Family
The Emervel family is differently designed. All products have the same HA content with 20 ng/ml. The products, however, are specified for their designated indications by the degree of cross-linking and by the grade of calibration. Two good clinical trials exist comparing the efficacy and safety to the Restylane/Restylane Perlane products (Ascher et al. 2011; Rzany et al. 2011). Furthermore, there is a unique case series where patients could be treated for a variety of indications with a variety of the Emervel products (Rzany et al. 2012).
The Allergan Juvéderm Family
The Allergan Voluma Family (aka Juvéderm Voluma Family)
These are the new products of Allergan. Compared to the Juvéderm products, the Voluma/Volbella/Volift fillers are cross-linked with shorter chains. Here we one good randomized clinical trials and some cases series (Callan et al. 2013; Jones et al. 2013). The Volbella case series is the case series on lip augmentation with the longest duration (e.g., 12 months) (Eccleston and Murphy 2012). Please note that as this is a newly designed HA filler, no final conclusion on the overall tolerability and safety can be made.
The Belotero Family
This is a product that was produced by Anteis and distributed by Merz, now with Merz having bought Anteis it is entirely in the hands of Merz. Like the abovementioned products, we have at least one good clinical trial here (Narins et al. 2010c).
The Teosyal Family
Again here we have a product with at least some clinical data behind it (Nast et al. 2011).
1.3.2.2.2 Products Without Good Clinical Data
Most available HA products in Europe do not have at least one good clinical trial in their background. They pretend to be as good as the products without clinical trials. However, caution is advisable.
1.3.2.2.3 Safety of HA Fillers
Hyaluronic acid is less allergenic than bovine collagen. Skin testing is not recommended. Although hyaluronic acids of human and of animal origins are identical in structure, immunological reactions in the recipient can be caused by residual proteins from the donor (avian or bacterial antigens) or from the cross-linking process.
For the products with good clinical trials besides the RCTs, several larger case series about safety are available. Lowe et al. (2001) reported 709 patients who were observed for a minimum of 1 year. Patients were treated with hyaluronic acid of avian or bacterial origin (patient cohort, follow-up study) between September 1996 and September 2000. The overall incidence of late inflammatory reactions (indurations, inflammation/erythema, abscess formation an average of 8 weeks after injection) is given as 0.42 % (3 out of 709 patients). Friedman et al. (2002) retrospectively reviewed the data of all unwanted effects of nonanimal hyaluronic acid from the Restylane family that were reported to the manufacturer between 1999 and 2000, worldwide (Europe, Australia, South America, and Asia). For 1999, based on 144,000 treatments, the incidence was calculated at 0.15 %; for 2000, based on approximately 262,000 treatments, the incidence of 0.06 % was given. Since the incidences reported by Lowe et al. (2001) and Friedman et al. (2002) are based on either patients returning to their private practice or voluntary reports, the real incidence might be higher.
In 2004, Andre evaluated the incidence of adverse reactions with nonanimal, stabilized hyaluronic acid between 1997 and 2001 using a questionnaire-based survey. Out of 12,344 syringes sold and 4,320 treated patients, 16 cases of immediate hypersensitivity and 18 cases of delayed reactions were recorded. The global risk of sensitivity was calculated at 0.8 %. Since 2000, the amount of protein in the raw product was decreased and the incidence of hypersensitivity reactions has been reported to be around 0.6 %. As 50 % of these reactions were immediate and resolved within less than 3 weeks, the risk of a strong but transient, delayed reaction is around 0.3 %. Four cases of sterile abscess were reported (Andre 2004). Again, although the data were quite systematically assessed, an underestimation of the real incidence cannot be ruled out.
Further case reports that are available describe in detail adverse reactions such as erythema, pruritus, edema, urticae, and papulocystic nodules after injection with hyaluronic acid preparations of various origins. Arterial embolization and exudative granulomatous reaction after treatment with hyaluronic acid of avian origin have also been reported (Fernandez-Acenero et al. 2003; Lombardi et al. 2004; Lowe 2003; Lupton and Alster 2000; Micheels 2001; Raulin et al. 2000; Shafir et al. 2000). In rare cases, a bluish discoloration might occur. This bluish discoloration may reflect a Tyndall phenomenon.
Not every product is similar. There appear to be products there with an increased risk of adverse reactions. How are we able to detect these products? Only by communicating adverse reactions among colleagues and to the authorities! In the Netherlands the sales of Hyacorp H-S and H1000 were temporarily stopped after several cases occurred with two products of the family (Skipr. Tot nu toe 25 klachten over rimpelfiller. Online in the Internet: http://www.skipr.nl/actueel/id12523-tot-nu-toe-25-klachten-over-rimpelfiller.html [2012-10-25]). At the moment (August 2013), these cases are still investigated by the Dutch authorities. However, the company decided to withdraw Hyacorp H-S 500, H1000, and Hyacorp L from the European market (mailing to doctors using Hyacorp in August 2013).
Key Points
Among the bacterial HAs, there is an easy way to distinguish between products with good clinical data and those without.
If you use a product without good clinical data, the risk for a not-so-good efficacy or an increased risk of inflammatory reactions is with the patient and the treating physician.
1.3.3 Combination of Hyaluronic Acid with Other Substances
1.3.3.1 Combination with Dextrans
The combination of hyaluronic acid, hydroxproylmethylcelluose and dextrans (dextranomers), marketed as Matridex, is thought to be more durable than other products. However, there is as yet no good clinical data on its efficacy and safety.
How safe is the addition of dextrans to an HA preparation? Or how safe are the HAs which are combined with dextrans? We do not have good clinical trials to answer these questions. However, we do have some case reports focusing on adverse reactions to these products (Huh et al. 2010; Massone et al. 2009). One patient developed after 5 weeks a delayed inflammatory reaction to Matridex injected in the glabellar fold that lasted more than a year. The patient was treated with oral doxycycline and intralesional injection of triamcinolone acetonide; this resulted in almost complete resolution of the lesion (Huh et al. 2010). The second patient was a 43-year-old woman who complained of multiple, painful, reddish, nonulcerated, hard nodules on both cheeks and periocular regions 4 weeks after the injection of Matridex. Histopathology showed a diffuse suppurative granulomatous reaction with the presence of multinucleate giant cells and many neutrophils involving the entire dermis (Massone et al. 2009).
1.3.3.2 Combination with Antioxidants
There are also HA products available where the HA is combined with, e.g., antioxidants. No good comparative trial data exists for these products. Therefore, it is not clear if these added substances have any clinically measurable effect at all.
Key Points
We do not have good clinical data on HAs in a fixed combination with dextrans or antioxidants.
Caution is therefore advisable as case reports of adverse reactions have been reported.
1.3.4 Alginates
At the end of 2009, a new filler, an alginate, which derives from brown algae was introduced to the market. Based on the results of the initial large cases series, the product looked very good. Erythema, swelling, and even hematomas seem to be less as for HA products (unpublished data presented at IMCAS Paris 2010).
In contrast to other filler, the alginate could be very easily injected. And this is probably what caused even experienced and trained injectors to inject too much in nonclinical investigated areas as the infraorbital hollow.
As some adverse events as nodule formation were reported specifically in areas as the infraorbital hollow and no antidote was available at that time, the filler was removed from the market (Schuller-Petrović et al. 2013).
Do’s
Watch out for this product. If reintroduced in the market, it could be (if an antidote is available) an interesting alternative in patients prone to erythema and edema after HA fillers.
Key Points
The efficacy and safety of alginates were supported by a large case series.
At the moment the filler is not available any more.
1.3.5 Poly-l-lactic Acid
Poly-l-lactid acid (PLLA) is a synthetic biodegradable material. It has an unique collagen stimulating quality. When injected into the dermis or subdermis, it gradually stimulates collagen formation and by this restructures the facial tissue, making it a facial volumizer. This is a gradual process, and the manufacturer recommends three initial treatment sessions, each approximately 6–8 weeks apart. After the three initial treatments, the results are supposed to last for up to 2 years and longer.
This product comes as a powder and needs to be diluted with sterile water several hours before injection. Although initially the recommended dilution for PLLA was 3 ml, the current recommendation is to dilute it in a volume of 5–9 ml or more. Most colleagues add an additional 2 ml of a local anesthetic to decrease injection pain. The correct recommendation from the SculpTraining Expert Group is 7 ml + 2 ml of a local anesthetic, making it a total of 9 ml. Furthermore, it is recommended to dilute the product at least 24 h before use. Even when administered using the correct injection technique and the higher dilution, in some cases the needle will block during the injection, at which point the syringe has to be withdrawn and the plunger retracted until the PLLA flows again.
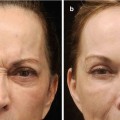
Before 2010, studies on the efficacy and safety of PLLA were based mainly on the treatment of HIV patients with drug-induced lipoatrophy (Moyle et al. 2004; Perry 2004; Valantin et al. 2003). Only case reports and case series existed for the use of PLLA for aesthetic indications (Rzany et al. 2004; Woerle et al. 2004). In 2010 a large clinical trial was published comparing PLLA to human collagen (Narins et al. 2010a) (n = 233). In this trial, at 3 months already the superiority of this product was shown compared to human collagen. The mean number of treatment sessions required per subject was 3.2 in the injectable PLLA group compared with 2.6 in the collagen group. The mean (SD) volume of injectable PLLA used per session for both nasolabial folds was as follows: session 1, 4.1 (1.1) ml; session 2, 3.5 (1.2) ml; session 3, 3.3 (1.2) ml; and session 4, 3.5 (1.1) ml. For human collagen, the mean (SD) volume used per session was as follows: session 1, 3.1 (1.1) ml; session 2, 2.1 (1.1) ml; session 3, 1.9 (1.1) ml; and session 4, 1.7 (1.0) ml. Importantly the correction with PLLA lasted over 13–25 months. This is the reason why most patients preferred this product (Brandt et al. 2011). However, the correction that could be achieved was less than you would see in HA trials (approximately 0.66–0.85 on a six-point scale – compared to approximately 1 on a five-point scale in HA trials (see above)) (Narins et al. 2010a).
Nodule formation is the main adverse reaction of this filler. Based on the HIV-lipodystrophy data, granulomatous reactions, described as palpable but invisible subcutaneous micronodules, were observed in 22 out of 50 (44 %) patients. In 6 of these 22 patients, the nodules disappeared at week 96. In that particular study, one vial of PLLA was diluted in a volume of 3–4 ml (Valantin et al. 2003).
The prevalence of nodule formation seems to be associated with the volume that was used for dilution. We have some indirect evidence that nodule formation can be reduced when an increased volume for dilution is used (Rossner et al. 2009a; Schierle and Casas 2011). Nevertheless, nodule formation still occurs and was highest in the hands (12.5 %) and the cheeks (7.2 %) (Palm et al. 2010). There are case reports of large solitary nodular masses, e.g., in the temporal region (Avery and Clifford 2010) as well as abscess formation. However, nodule formation is in most patients temporary and will decrease over time (Sperling et al. 2010). Therefore, these nodules should not be too aggressively treated.
Do’s
Always dilute the PLLA with at least 5–7 ml at least 24 h before treatment.
Add an additional 2 ml of a local anesthetic before injection.
Patients with severe elastosis might benefit from a topical co-treatment with 0.05 % tretinoin.
Don’ts
Do not inject PLLA too superficially in elastotic or naturally very thin skin as otherwise you might end up with small superficial bumps.
Do not inject PLLA in hyperdynamic areas as the lips.
Do not inject PLLA in patients with active autoimmune diseases as rheumatoid arthritis (see Chap. 8).
Do not inject PLLA in patients which might be subject to interferon therapy (if foreseeable) – they might have an increased risk for nodule formation.
Key Points
This is a product where we have at least a one good clinical trial and several case series available.
PLLA needs to be injected several times to obtain a clinically relevant result. At least three treatment sessions should be planed, each session at least 4–6 weeks apart from the other.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree