Fig. 25.1
Bacitracin (NACS)
25.4 Benzocaine (EBS, NACS) (Fig. 25.2)
Ethyl 4-aminobenzoate
CAS Registry Number [94–09–7]
Benzocaine is a local anesthetic derived from p-aminobenzoic acid. Cross-reactions with other p-amino compounds such as PPD have been reported.
Suggested Reading
Gail H et al. Adverse reactions to local anesthetics: analysis of 197 cases. J Allergy Clin Immunol. 1996;97:933–7.
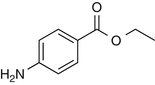

Fig. 25.2
Benzocaine (EBS, NACS)
25.5 Benzophenone-3 (NACS, CBS) (Fig. 25.3)
Oxybenzone
CAS Registry Number [131–57–7]
Benzophenones (BZPs), and substituted BZP numbered 1–12, are photo-screen agents widely used in sunscreens and in cosmetics, such as “antiaging” creams and hair sprays and shampoos, but also in paints and plastics. BZP-3 is used as a direct sunscreen agent and in antiaging creams. Cross-reactivity is expected in an average of one in four patients photoallergic to ketoprofen.
Suggested Reading
Matthieu L et al. Contact and photocontact allergy to ketoprofen. The Belgian experience. Contact Dermatitis. 2004;50:238–41.
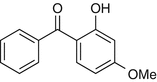

Fig. 25.3
Benzophenone-3 (NACS, CBS)
25.6 Benzoyl Peroxide (CBS) (Fig. 25.4)
CAS Registry Number [94–36–0]
Benzoyl peroxide is an oxidizing agent widely used in acne topical therapy. It is also used as a polymerization catalyst of dental or industrial plastics, as a bleaching agent of flours, oils, fats, and waxes. Irritant or allergic dermatitis may affect workers in the electronics and plastics (epoxy resins and catalysts) industries, electricians, ceramic workers, dentists and dental technicians, laboratory technicians, bakers, and acne patients.
Suggested Reading
Rustemeyer T, Frosch PJ. Occupational skin diseases in dental laboratory technicians. (I). Clinical picture and causative factors. Contact Dermatitis. 1996;34:125–33.
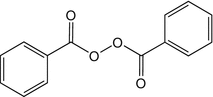

Fig. 25.4
Benzoyl peroxide (CBS)
25.7 Benzyl Salicylate (CBS) (Fig. 25.5)
Benzyl-o-hydroxybenzoate, 2-hydroxybenzoic acid phenylmethyl ester
CAS Registry Number [118–58–1]
Benzyl salicylate is used in perfumery and in sunscreen preparations as a fixative agent. As a fragrance sensitizer, it has to be listed by name in cosmetic preparations in the EU.
Suggested Reading
Larsen W et al. Fragrance contact dermatitis: a worldwide multicenter investigation (Part I). Am J Contact Dermatitis. 1996;7:77–83.
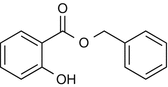

Fig. 25.5
Benzyl salicylate (CBS)
25.8 2-Bromo-2-nitropropane-1,3-diol (NACS, CBS) (Fig. 25.6)
Bronopol
CAS Registry Number [52–51–7]
Bronopol is a preservative sometimes considered as a formaldehyde releaser. It was reported to be an allergen in cosmetics and cleaning agents, in dairy workers and in a gel used for ultrasound examination.
Suggested Reading
Wilson CL, Powell SM. An unusual cause of allergic contact dermatitis in a veterinary surgeon. Contact Dermatitis. 1990;23:42–3.


Fig. 25.6
2-Bromo-2-nitropropane-1,3-diol (NACS, CBS)
25.9 Budesonide (EBS, NACS) (Fig. 25.7)
CAS Registry Number [51333–22–3]
(R)-Budesonide/CAS Registry Number [51372–29–3]; (S)-Budesonide/CAS Registry Number [51372–28–2]
Budesonide is a mix of two diastereoisomers. The (R)-budesonide is considered to be a marker of the B group of corticosteroids (amcinonide, budesonide, desonide or prednacinolone, flunisolide, fluocinolone and its acetonide, fluocinonide, fluclorolone and its acetonide, halcinonide, and acetonide, benetonide, diacetate, and hexacetonide of triamcinolone). The (S)-budesonide is considered to be a marker of the D2 group of corticosteroids (hydrocortisone 17-butyrate, hydrocortisone 17-valerate, hydrocortisone aceponate, methylprednisolone aceponate, and prednicarbate).
Suggested Reading
Lepoittevin JP, Drieghe J, Dooms-Goossens A. Studies in patients with corticosteroid contact allergy. Understanding cross-reactivity among different steroids. Arch Dermatol. 1995;131:31–7.
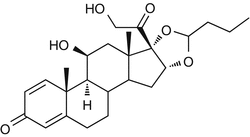

Fig. 25.7
Budesonide (EBS, NACS)
25.10 2-tert-Butyl-4-methoxyphenol/BHA (CBS) (Fig. 25.8)
Butylated hydroxyanisole
CAS Registry Number [25013–16–5]
BHA is an antioxidant widely used in cosmetics and food. It was also reported to induce sensitization in caterers.
Suggested Reading
Acciai MC et al. Allergic contact dermatitis in caterers. Contact Dermatitis. 1993;28:48.
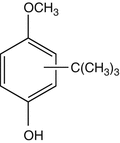

Fig. 25.8
2-tert-Butyl-4-methoxyphenol/BHA (CBS)
25.11 4-tert-Butylphenol formaldehyde Resin/PTBP (EBS, NACS, CBS) (Fig. 25.9)
para-tert-Butylphenol
CAS Registry Number [98–54–4]
The polycondensate p-tert-butylphenol formaldehyde resin (PTBPFR) is obtained by reaction of p–tert-butylphenol with formaldehyde. Major occupational sources are neoprene glues and adhesives in industry, in the shoemaking and leather industries, or in car production. It is also used as a preservative in box and furniture manufacture and in the production of casting molds, car brake linings, insulated electrical cables, adhesives, printing inks, and paper laminates. p–tert-Butylphenol seems to be the main sensitizer.
Suggested Reading
Tarvainen K. Analysis of patients with allergic patch test reactions to a plastics and glue series. Contact Dermatitis. 1995;32:346–51.
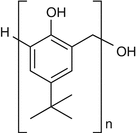

Fig. 25.9
4-tert-Butylphenol formaldehyde resin/PTBP (EBS, NACS, CBS)
25.12 Cananga odorata Oil
Ylang-ylang oil
CAS Registry Number [8006–81–3]
This essential oil is obtained from flowers of Cananga odorata Hook. F. and Thoms. It is mainly used as a fragrance material in fine perfumes but also in aromatherapy and can therefore induce occupational contact dermatitis. The oil contains several potential allergens such as isoeugenol and derivatives of geraniol and linalool (see FMI).
Suggested Reading
Romaguera C, Vilaplana J. Occupational contact dermatitis from ylang-ylang oil. Contact Dermatitis. 2000;43:251.
25.13 Carba Mix (NACS, CBS) (Fig. 25.10)
This mix contains the following three allergens: diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethyldithiocarbamate. These chemicals are used as fungicides and pesticides and also in the manufacture of many rubber products. One can get in contact with these substances when using, wearing, or handling rubber products at work or at home.
(a)
1,3-Diphenylguanidine/CAS Registry Number [102–06–7]
Diphenylguanidine is a rubber sensitizer that can induce both immediate- and delayed-type contact allergy. Occupational exposure concerns finished rubber items and the rubber manufacturing industry. The most frequent occupational categories are metal industry, homemakers, health services and laboratories, and the building industry.
(b)
Zinc bis-Dibutyldithiocarbamate/CAS Registry Number [136–23–2]
It is used as a vulcanization accelerator; it can also be contained in paints, glue removers, and anticorrosive products.
(c)
Zinc bis-Diethyldithiocarbamate/CAS Registry Number [14324–55–1]
It is a rubber component used as a vulcanization accelerator. Oxidation of this carbamate derivative leads to tetraethylthiuram disulfide.
Suggested Reading
Condé-Salazar L et al. Type IV allergy to rubber additives: a 10-year study of 686 cases. J Am Acad Dermatol. 1993;29:176–80.
Chipinda I et al. Zinc diethyldithiocarbamate allergenicity: potential haptenation mechanisms. Contact Dermatitis. 2008;59:79–89.
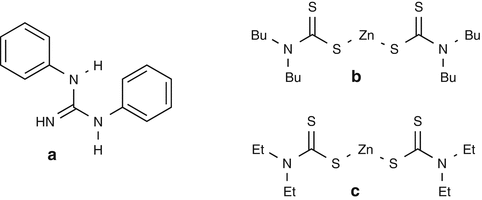

Fig. 25.10
Carba mix (NACS, CBS)
25.14 Chloroxylenol/PCMX (NACS, CBS) (Fig. 25.11)
4-Chloro-3,5-dimethylphenol
CAS Registry Number [88–04–0]
Chloroxylenol is a broad-spectrum antimicrobial chemical compound used to control bacteria, algae, fungi, and viruses. It is primarily used in household products and topical medicine.
Suggested Reading
Berthelot C, Zirwas MJ. Allergic contact dermatitis to chloroxylenol. Dermatitis. 2006;17:156–9.
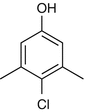

Fig. 25.11
Chloroxylenol/PCMX (NACS, CBS)
25.15 Cinnamal (NACS, CBS) (Fig. 25.12)
Cinnamic aldehyde, cinnamaldehyde, 3-phenyl-2-propenal
CAS Registry Number [104–55–2]
This chemical is used as a fragrance in perfumes and as a flavoring agent in soft drinks, ice creams, toothpastes, pastries, chewing gum, etc. It can induce both contact urticaria and delayed-type reactions. It can be responsible for dermatitis in the perfume industry or in food handlers. Cinnamic aldehyde is contained in “fragrance mix I” and has to be mentioned by name in cosmetics within the EU.
Suggested Reading
Seite-Bellezza D et al. Contact urticaria from cinnamic aldehyde and benzaldehyde in a confectioner. Contact Dermatitis. 1994;31:272–3.
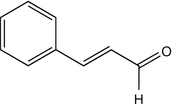

Fig. 25.12
Cinnamal (NACS, CBS)
25.16 Clioquinol (EBS) (Fig. 25.13)
Chloroiodoquine, 5-chloro-7-iodoquinolin-8-ol
CAS Registry Number [130–26–7]
Clioquinol is an antifungal and antiprotozoal drug. It can be incorporated in many topical creams and ointments to treat skin conditions in which an anti-infective agent is required. The oral administration of clioquinol has resulted in a generalized eruption in individuals allergic to this chemical.
Suggested Reading
Morris SD et al. Comparative frequency of patch test reactions to topical antibiotics. Br J Dermatol. 2002;146:1047–51.
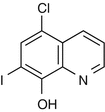

Fig. 25.13
Clioquinol (EBS)
25.17 Cocamide DEA (CBS) (Fig. 25.14)
Coconut diethanolamide, coconut oil fatty acid diethanolamide, N,N-bis(2-hydroxyethyl)coco fatty acid diethanolamide, cocoyl diethanolamide
CAS Registry Number [68603–42–9]
Cocamide DEA, manufactured from coconut oil, is widely used in industry and at home as a surface-active agent. It is contained in hand gels, handwashing soaps, shampoos, and dishwashing liquids for its foam-producing and stabilizing properties and in metalworking fluids and polishing agents as an anticorrosion inhibitor.
Suggested Reading
Fowler JF Jr. Allergy to cocamide DEA. Am J Contact Dermatitis. 1998;9:40–1.
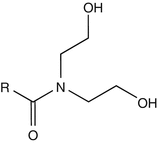

Fig. 25.14
Cocamide DEA (CBS)
25.18 Cocamidopropyl Betaine (NACS) (Fig. 25.15)
Cocoamphodipropionate, cocamidopropyl dimethyl glycine, cocoamphocarboxypropionate, cocoyl amide propylbetaine
CAS Registry Numbers [61789–40–0], [83138–08–3], [86438–79–1]
Cocamidopropyl betaine is a pseudo-amphoteric zwitterion detergent derived from long-chain alkyl betaines. It is available from many suppliers under more than 50 trade names. Exposure occurs via rinse-off products such as liquid soaps, shampoos, and shower gels but also via leave-on products such as roll-on deodorants. Occupational sources are mainly in hairdressing. The first synthesis step consists of the reaction of coconut fatty acids with 3-dimethylaminopropylamine, giving cocamidopropyl dimethylamine. This amidoamine is converted into cocamidopropyl betaine by reaction with sodium monochloroacetate. Both dimethylaminopropylamine and cocamidopropyl dimethylamine are thought to be the sensitizers.
Suggested Reading
McFadden JP et al. Clinical allergy to cocamidopropyl betaine: reactivity to cocamidopropylamine and lack of reactivity to 3-dimethylaminopropylamine. Contact Dermatitis. 2001;45:72–4.
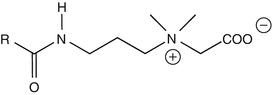

Fig. 25.15
Cocamidopropyl betaine (NACS)
25.19 Colophonium (EBS, NACS, CBS) (Fig. 25.16)
Abietic acid/CAS Registry Number [514–10–3]
Abietic acid is probably the major source of allergens in colophony or rosin by way of air oxidation. The formation of highly sensitizing hydroperoxides has been demonstrated, and therefore the detection of abietic acid in a material indicates that allergenic components of colophony are present.
Suggested Reading
Karlberg AT et al. Is abietic acid the allergenic component of colophony? Contact Dermatitis. 1985;13:209–15.
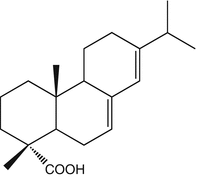

Fig. 25.16
Colophonium (EBS, NACS, CBS)
25.20 Compositae Mix II (NACS) (Fig. 25.17)
This mix was introduced as a complement of the sesquiterpene lactone mix for the detection of Compositae dermatitis. It is made of parthenolide and a sesquiterpene lactone and of five natural extracts: Tanacetum vulgare L., Arnica montana L., Chamomilla romana (Anthemis nobilis L.), Matricaria chamomilla L., and Tanacetum millefolium (L.) Tzvelev.
Parthenolide/CAS Registry Number [20554–84–1]
Parthenolide is a sesquiterpene lactone found in Asteraceae/Compositae such as feverfew (Tanacetum parthenium Schultz-Bip.) or congress grass (Parthenium hysterophorus L.).
Suggested Reading
Paulsen E et al. Compositae dermatitis in a Danish dermatology department in one year (I). Contact Dermatitis. 1993;29:6–10.
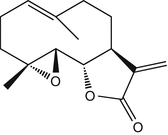

Fig. 25.17
Parthenolide (NACS)
25.21 Decyl Glucoside (CBS) (Fig. 25.18)
Decyl D-glucoside, decyl-beta-D-glucopyranoside
CAS Registry Number [58846–77–8]
Decyl glucoside belongs to the alkyl glucoside family and is obtained by condensation of the fatty alcohol decyl alcohol and a D-glucose polymer. This nonionic surfactant and cleansing agent has been widely used for several years in rinse-off products such as shampoos, hair dyes and colors, and soaps due to its foaming power and good tolerance. Decyl glucoside is also employed in leave-on products such as no-rinse cleansing milks, lotions, and several sunscreen agents.
Suggested Reading
Goossens A et al. Glucosides as unexpected allergens in cosmetics. Contact Dermatitis. 2003;48:164–6.
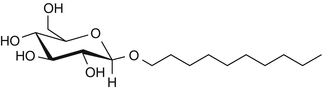

Fig. 25.18
Decyl glucoside (CBS)
25.22 Diazolidinyl Urea (NACS, CBS) (Fig. 25.19)
Germall II
CAS Registry Number [78491–02–8]
Diazolidinyl urea, a formaldehyde releaser, is contained mainly in cosmetics and toiletries, but can also be found in barrier creams.
Suggested Reading
Van Hecke E, Suys E. Where next to look for formaldehyde? Contact Dermatitis. 1994;31:268.
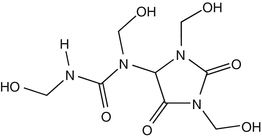

Fig. 25.19
Diazolidinyl urea (NACS, CBS)
25.23 Dimethylol Dihydroxy Ethylene Urea (NACS) (Fig. 25.20)
CAS Registry Number [136–84–5]
Dimethylol ethylene urea is an organic compound derived from formaldehyde and urea. It is used for treating cellulose-based fabrics to avoid wrinkle formation. The chemical can release formaldehyde.
Suggested Reading
Metzler-Brenckle L. Rietschel RL. Patch testing for permanent-press allergic contact dermatitis. Contact Dermatitis. 2002;46:33–7.
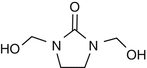

Fig. 25.20
Dimethylol dihydroxy ethylene urea (NACS)
25.24 Disperse Blue Mix (NACS, CBS) (Fig. 25.21)
This mix contains two of the most often incriminated disperse textile dyes: disperse blue 106 and 124. Concomitant reactions between these dyes are due to their chemical similarities. Both are used in synthetic fibers.
(a)
Disperse blue 106/CAS Registry Number [74339–69–8]
(b)
Disperse blue 124/CAS Registry Number [15141–18–1]
They are used in synthetic fibers.
Suggested Reading
Mota F et al. An outbreak of occupational textile dye dermatitis from Disperse Blue 106. Contact Dermatitis. 2000;43:235–6.
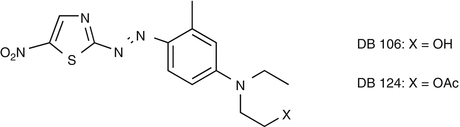

Fig. 25.21
Disperse blue mix (NACS, CBS)
25.25 Disperse Orange 3 (CBS) (Fig. 25.22)
CAS Registry Number [730–40–5]
Disperse orange 3 is an azo dye reported to induce contact dermatitis in the textile industry. It is positive in a great majority of PPD-positive individuals, because of metabolism in the skin into PPD. Disperse orange 3 can also be found in some semipermanent hair dyes.
Suggested Reading
Soni BP, Sherertz EF. Contact dermatitis in the textile industry: a review of 72 patients. Am J Contact Dermatitis. 1996;7:226–30.
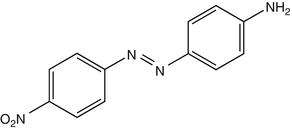

Fig. 25.22
Disperse orange 3 (CBS)
25.26 Disperse Yellow 3 (CBS) (Fig. 25.23)
CAS Registry Number [2832–40–8]
This azo dye is responsible for textile dermatitis from stockings and occupational contact dermatitis in the textile industry. It can be found in some semipermanent hair dyes.
Suggested Reading
Condé-Salazar L et al. Contact dermatitis in hairdressers: patch test results in 379 hairdressers (1980–1993). Am J Contact Dermatitis. 1995;6:19–23.
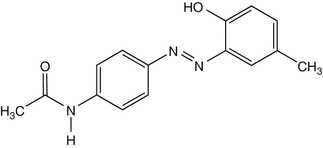

Fig. 25.23
Disperse yellow 3 (CBS)
25.27 DMDM Hydantoin (NACS, CBS) (Fig. 25.24)
1,3-Dimethylol-5,5-dimethylhydantoin, Glydant™
CAS Registry Number [6440–58–0]
DMDM hydantoin is an antimicrobial formaldehyde releaser preservative belonging to a class of compounds known as hydantoins. It is used in the cosmetics industry and found in products like shampoos, hair conditioners, hair gels, and skin care products.
Suggested Reading
de Groot AC et al. Patch test reactivity to DMDM hydantoin. Relationship to formaldehyde allergy. Contact Dermatitis. 1988;18:197–201.
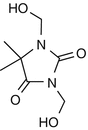

Fig. 25.24
DMDM hydantoin (NACS, CBS)
25.28 Ethyl Acrylate (NACS, CBS) (Fig. 25.25)
CAS Registry Number [140–88–5]
Acrylates are esters of acrylic acid, and occupational contact allergies from acrylates have frequently been reported in workers exposed to the glues, as well as dental workers and beauticians.
Suggested Reading
Rustemeyer T, Frosch PJ. Occupational skin diseases in dental laboratory technicians. (I). Clinical picture and causative factors. Contact Dermatitis. 1996;34:125–33.
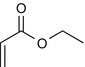

Fig. 25.25
Ethyl acrylate (NACS, CBS)
25.29 Ethylenediamine (NACS, CBS) (Fig. 25.26)
CAS Registry Number [107–15–3]
Ethylenediamine is used in numerous industrial processes as a solvent for casein or albumin, as a stabilizer in rubber latex, and as a textile lubricant. It can be found in epoxy resin hardeners, cooling oils, fungicides, and waxes. Contact dermatitis from ethylenediamine is almost exclusively due to topical drugs. Ethylenediamine can cross-react with triethylenetetramine and diethylenetriamine.
Suggested Reading
Sasseville D, Al-Khenaizan S. Occupational contact dermatitis from ethylenediamine in a wire-drawing lubricant. Contact Dermatitis. 1997;3:228–9.


Fig. 25.26
Ethylenediamine (NACS, CBS)
25.30 Epoxy Resins of the Bisphenol A Type (EBS, NACS, CBS) (Fig. 25.27)
These resins are synthesized from bisphenol A and epichlorhydrin. Hardeners are added, such as amines (ethylenediamine, diethylenetriamine, triethylenetetramine, isophoronediamine, triethylenetriamine, and 4,4-diaminodiphenylmethane) and acid anhydrides (phthalic anhydride). Reactive diluents may be added, such as allyl glycidyl ether, butanediol diglycidyl ether, n-butyl glycidyl ether, o-cresyl glycidyl ether, hexanediol diglycidyl ether, neopentyl glycol diglycidyl ether, phenyl glycidyl ether, glycidyl ester of synthetic fatty acids, and glycidyl ether of aliphatic alcohols.
Bisphenol A Diglycidyl Ether (DGEBA)/CAS Registry Number [1675–54–3]
Most epoxy resins result from polymerization of bisphenol A diglycidyl ether (BADGE). Delayed hypersensitivity is caused by the low-molecular-weight monomer BADGE (Mol. Wt. 340 g/mol), the dimer having much a lower sensitization power.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







