Fig. 1.1
Immunohistochemistry of the skin with S100. The skin consists of the corneum, epidermis, and dermis. The epidermis contains Langerhans cells, and the dermis contains dermal dendritic cells, which serve as antigen presenting cells
It was recently demonstrated that there are about 20 billion T cells in the skin of an adult human, bearing markers that identify them as skin homing memory T cells (CD45RO/CLA/CCR4) [1]. Not only are there twice as many T cells in skin as in blood, but the number of memory T cells with a skin homing phenotype is more than 20 times the number of those in the blood. In addition, LCs localize in the epidermis as antigen presenting cells at a density of 1000 per mm2, suggesting that the skin is an important immune organ.
As a result of immune responses to external antigens, inflammatory skin diseases can be induced: urticaria by oral intake of allergens, including egg and fish; contact dermatitis by haptens, including metals and urushiol; and atopic dermatitis by proteins, including mites, house dust, and pollen (Fig. 1.2) [2–5].
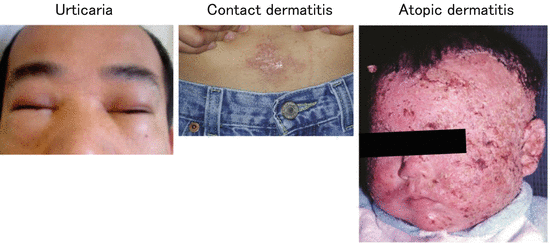

Fig. 1.2
Clinical manifestations of inflammatory skin diseases
1.2 Concept of SALT (Skin Associated Lymphoid Tissue)
Immune function is not limited to the skin. In submucosal areas, for example, specific sentinel lymphoid tissues called mucosa-associated lymphoid tissues (MALT) serve as peripheral antigen presentation sites [6]. By analogy, the concept of skin-associated lymphoid tissue (SALT) was proposed in the early 1980s based on the discovery that (1) the cutaneous microenvironment can accept, process, and present antigens, (2) the peripheral lymph nodes (LNs) can accept immunogenic signals derived from the skin, (3) subsets of T cells exhibit a differential affinity for skin, and (4) the acquisition of this affinity by T cells is determined by resident cutaneous cells [7].
On the other hand, there are distinct functional differences between MALT and SALT. MALT contains significant numbers of B cells and forms lymphoid follicles, whereas virtually all lymphocytes within the skin are T cells. MALT lymphoid follicles are surrounded by T-cell–rich areas in which high endothelial venules (HEVs) are embedded and serve as entry points for naïve T cells. Therefore, MALT provides a field for antigen presentation to naïve T cells as well as other secondary lymphoid organs. SALT, in contrast, contains no HEVs, and the T cells in skin are memory T cells rather than naïve T cells. Therefore, skin-draining LNs are necessary for the priming of naïve T cells to foreign antigens that have invaded through the skin.
The T-cell homing system is tightly regulated by the expression of adhesion molecules and the chemokine receptors called addressins. Certain T cell subsets have a high affinity for the skin and the gut as well as for secondary lymphoid organs (Table 1.1, Fig. 1.3). Thus it is clear that defending the outermost membranes, namely, the skin and the gut, is a high priority for the immune system.
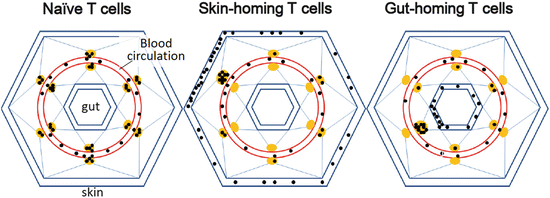
Table 1.1
Receptors involved in tissue-specific homing
Cell type | Receptor | Ligand | |
|---|---|---|---|
Peripheral LNs | Naive T, TCM | CCR7 | CCL19, CCL21 |
Naive T, TCM | CD62L | sLex | |
Gut | TEM | CCR9 | CCL25 |
TEM | α4β7-Integrin | MAdCAM-1 | |
Skin | TEM | CLA | E-selectin |
TEM (Th1) | CXCR3 | CXCL9, CXCL10 | |
TEM (Th2) | CCR4 | CCL17, CCL21 | |
TEM (Th2) | CCR10 | CCL27, CCL28 | |
TEM (Th2) | CCR8 | CCL8 |

Fig. 1.3
Tissue-specific homing ability of T cells. Three distinct homing systems to the secondary lymphoid organs, skin, and gut. Solid black and brown circles denote T cells and lymph nodes, respectively
1.3 Immune Reactions to Foreign Antigens
Antigen presentation to T cells is essential for the induction of adaptive immunity . This event takes place not solely in the LNs where naïve T cells are primed but also in the skin where memory T cells are activated.
Upon protein antigen exposure, DCs acquire antigens and stimulate the proliferation of T cells to induce distinct T helper cell responses to external pathogens [3]. In mouse skin, there are at least three subsets of DCs [8–10]: LCs in the epidermis and Langerin-positive and Langerin-negative DCs in the dermis (Langerin+ dermal DCs and Langerin− dermal DCs, respectively).
It has been reported that, when epicutaneously applied, large molecules such as protein antigens are above the size-selective barrier known as the tight junction (Fig. 1.4), and that activated LCs extend their dendrites through the tight junction to take up antigens [11]. Topically applied haptens, on the other hand, penetrate into the dermis.
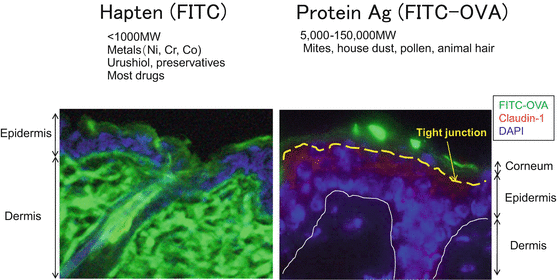

Fig. 1.4
Distribution of haptens and protein antigens upon epicutaneous application. After the epicutaneous application of green-fluorescent hapten (FITC) as a hapten and FITC-ovalbumin (FITC-OVA) as a protein antigen, FITC penetrates into the dermis whereas FITC-OVA is retained at the corneum
1.3.1 Immune Reactions to Haptens
Haptens are external antigens that easily penetrate into the dermis (Fig. 1.4). As is well known, a single hapten application induces a classic delayed-type hypersensitivity called the contact hypersensitivity (CHS) response, which is mediated by IFN-γ−producing CD8+ (Tc1) and CD4+ T (Th1) cells (Fig. 1.5) [3].
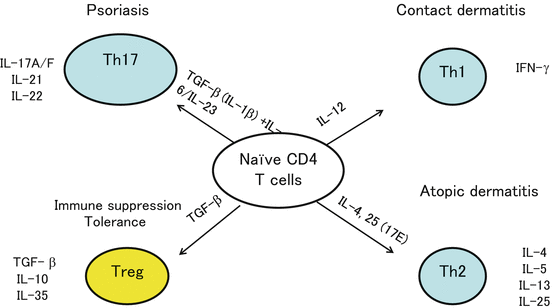

Fig. 1.5
T cell subsets. CD4+ helper T (Th) cells have at least three subtypes, Th1, Th2, and Th17, that are involved in the pathogenesis of contact dermatitis, AD/urticaria, and psoriasis, respectively. These Th subtypes are induced by specific cytokine conditions. Regulatory T cells (Treg), on the other hand, are localized in the skin where they play an important role in maintaining homeostasis and terminating a variety of skin immune responses
LCs have long been regarded as essential antigen-presenting cells for the establishment of sensitization in hapten-induced CHS, but this concept is now being challenged by recent analyses using LC ablation murine models [12]. In the development of CHS to haptens, Langerin-negative dermal DCs play a major role, whereas LCs and Langerin-positive dermal DCs play a compensatory role [13].
On the other hand, repeated applications of haptens (2,4,6-trinitrochlorobenzene; TNCB) induce atopic dermatitis-like skin lesions [14] by causing a shift from Th1- to Th2-mediated cutaneous inflammation with elevated IL-4 expression, eosinophil infiltration in the skin, and elevated hapten-specific serum IgE levels [14]. At present, which class of cells mediates the shift from Th1 to Th2 remains a topic of debate. One of the candidate classes appears to be basophils, which express MHC class II and IL-4 in the draining LNs [15] (Fig. 1.6).
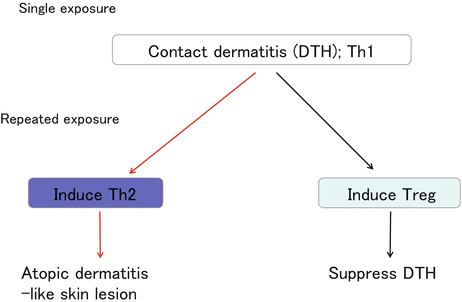

Fig. 1.6
Dynamics of cutaneous immune responses. A single hapten elicitation induces Th1-mediated delayed-type hypersensitivity (DTH), also known as contact dermatitis/contact hypersensitivity. Even during the contact dermatitis response, Treg accumulate in the skin and suppress DTH responses. Repeated hapten elicitation induces Th2 conditions in the skin, which are characteristic of atopic dermatitis
It has also been reported that regulatory T cells (Treg) accumulate in the skin during CHS [16] and that Treg suppress both the sensitization and the elicitation of the CHS response [17–19]. In addition, IL-10 is induced in the repeat hapten application-induced chronic CHS model [20]. These findings demonstrate that chronic antigen exposure induces Treg accumulation in the skin (Fig. 1.6). Clinically, topical immunotherapy with squaric acid dibutylester (SADBE) is effective for the treatment of alopecia areata [21]. It remains unclear how SADBE controls this autoimmune disease, but the accumulation of Treg in chronically hapten-exposed skin may play an important role (Fig. 1.5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








