(1)
Dermatologische Praxis & Haarcenter, Wallisellen (Zürich), Switzerland
The human body experiences a powerful gravitational pull in the direction of hope. That is why the patient’s hopes are the physician’s secret weapon. They are the hidden ingredients in any prescription.Norman Cousins (1915–1990)
Few dermatologic problems carry as much emotional overtones as the complaint of hair loss. The best way to alleviate the emotional distress related to hair loss is to effectively treat it. As with any medical problem, the patient complaining of hair loss requires a comprehensive medical and drug history, physical examination of the hair and scalp, and appropriate laboratory evaluation to identify the cause.
Prerequisite for delivering appropriate therapy is an understanding of the underlying pathologic dynamics. Once the diagnosis is certain, therapy appropriate for that diagnosis is likely to control the problem.
Although testing medical interventions for efficacy had existed since the time of Avicenna’s The Canon of Medicine in the eleventh century, it was only in the twentieth century that this effort evolved to impact almost all fields of health care and policy. In 1967, American physician and mathematician Alvan R. Feinstein published his seminal work Clinical Judgment, which together with Archie Cochrane’s celebrated book Effectiveness and Efficiency (1972) led to an increasing acceptance of clinical epidemiology and controlled studies during the 1970s and 1980s and prepared the way for the institutional development of EBM in the 1990s. EBM seeks to assess the strength of the evidence of risks and benefits of diagnostic tests and treatments, using techniques from science, engineering, and statistics, such as the systematic review of medical literature, meta-analysis, risk–benefit analysis, and randomized controlled trials.
EBM aims for the ideal that healthcare professionals should make conscientious, explicit, and judicious use of the best available evidence gained from the scientific method to clinical decision-making.
As EBM guidelines on hair loss are rare, a European consensus group was recently constituted to develop guidelines for treatment of androgenetic alopecia. The European consensus group conducted a systematic literature review in Medline, Embase, and Cochrane databases until August 2008. 1,370 publications were found and 51 added by hand search. 85 publications fulfilled the following inclusion criteria for the guideline: prospective study with a number of patients ≥20 (no minimal patient number required in twin studies), age ≥12 years, and with confirmed diagnosis of androgenetic alopecia (diagnosis either clinically or by further diagnostic evaluations, e.g., trichogram, TrichoScan, biopsy). Objective outcome measure of efficacy described for drug therapy was mean change from baseline hair count in target area or measurement of hair growth/loss in target area by global photography.
The guideline revealed excellent evidence levels for the therapeutic use of topical minoxidil and of oral finasteride, low evidence levels for hormonal treatments (in women), and insufficient, or lacking, evidence for a broad panel of miscellaneous treatments available claiming effectiveness for treatment of male or female androgenetic alopecia (Table 8.1).
Table 8.1
Overview of miscellaneous treatments with insufficient or lacking evidence for efficacy and proposed mechanisms of action in treatment of hair loss
Promotion of hair regrowth | Amino acids Iron supplements in the absence of deficiency Vitamins (biotin, niacin derivates) Proanthocyanidins Millet seed (silicic acid, amino acids, vitamins, minerals) Marine extract and silicea component Chinese herbals Ginkgo biloba Aloe vera Ginseng Bergamot Hibiscus Sorphora Caffeine Melatonin Retinoids Cyclosporine Electromagnetic/electrostatic field Low-level laser |
Improved perifollicular vascularization | Prostaglandins (viprostol, latanoprost) Aminexil Glyceroloxyesters and silicium Minerals Niacin derivatives Mesotherapy |
DHT inhibitory activity | Saw palmetto ß-sitosterol Polysorbate 60 Green tea Cimicifuga racemosa |
Anti-inflammatory activity | Ketoconazole Zinc pyrithione Corticosteroids |
Improved hair nutrition | Vitamins (biotin, niacin derivates) Trace elements (zinc, copper) |
Others | Botulinum toxin |
Although EBM is becoming regarded as the gold standard for clinical practice, there are a number of limitations of its use.
The limited success of evidence-based therapies points to a more important complexity of hair loss.
EBM guidelines do not remove the problem of extrapolation to different patient populations or longer time frames, and certain groups have been under-researched, such as people with comorbid diseases. Finally, EBM applies to groups of people, but this does not preclude clinicians from using their personal experience in deciding what is best for the individual at hand.
Therefore, good medical practice means integrating personal clinical expertise with the best available external clinical evidence from EBM.
By approaching the hair loss patient in a methodical way, commencing with objects the simplest and easiest to recognize, and ascending step by step to the knowledge of the more complex, an individualized treatment plan can be designed.
One must remain open-minded (1) for the possibility of a multitude of cause-relationships underlying hair loss and (2) to consider combination regimens for therapy that may act synergistic to enhance hair growth and quality. Both are too complex to be adequately included by the EBM method.
8.1 Impact of Seasonality of Hair Growth and Shedding
In 823 otherwise healthy women with telogen effluvium during an observational period of 6 years, Kunz et al. demonstrated the existence of an overall annual periodicity in the growth and shedding of hair, manifested by a maximal proportion of telogen hairs in July. Taking a scalp hair telogen phase duration of approximately 100 days into account, one would expect shedding of these hairs by autumn.
The authors pointed out that existence of seasonal fluctuations in hair growth and shedding complicates the assessment of pharmacological effects. Awareness of these fluctuations is prerequisite to providing the correct cause and prognosis to the patient and ensuring patient compliance with therapy and has potentially serious implications for investigations with new hair growth-promoting agents.
Depending on the stage of periodicity in growth and shedding of hair for a particular subject, the heterogeneity of included subjects relating to the season may be enough to distort clinical efficacy results of an investigational agent. In the active stage of seasonal telogen effluvium, the involved hair follicles would probably fail to respond to the therapeutic agent, which may cause a false-negative result. In the recovery stage, the increased amounts of spontaneous hair regrowth might be interpreted falsely as a positive result.
In her inaugural dissertation (University of Zurich, 2015), Hotzenköcherle Trüeb demonstrated the impact of seasonality of hair growth and shedding on study results through inhomogeneous inclusion of study patients in relation to the season. In a preceding double-blind placebo-controlled study using combining epiluminescence microscopy and digital imaging, efficacy of a specific oral hair growth-promoting agent for treating telogen effluvium in otherwise healthy women was demonstrated, and this is independent of patient age. Therefore, an open-label pilot study with the same agent was performed in 5 women aged between 64 and 84 years, which confirmed the former results with increase and normalization of anagen rates within 3 months of treatment (unpublished data). To further verify efficacy of the agent in women aged 60 years and more, Hotzenköcherle Trüeb performed a double-blind placebo-controlled study in 36 patients for a duration of 6 months. Unexpectedly, placebo showed similar improvement of hair growth as compared to active compound. A further subanalysis of patient data demonstrated that the result was falsified through inhomogeneous inclusion of study patients in relation to the season, with a cluster of patients on placebo profiting from the spontaneous recovery phase of seasonal hair growth and shedding (Fig. 8.1a–d).
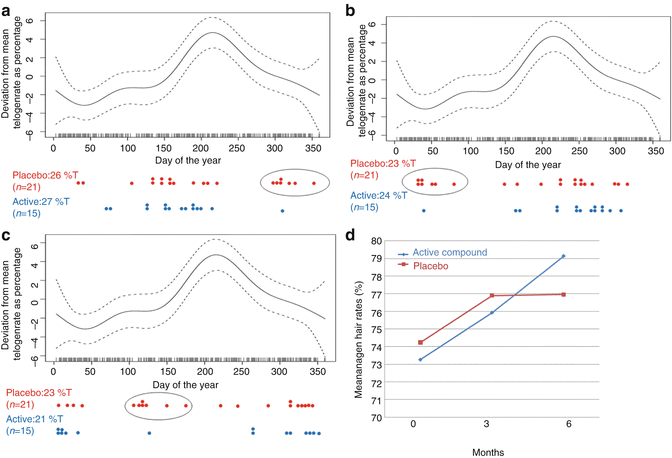

Fig. 8.1
(a–d) Inhomogeneous inclusion of study patients in relation to periodicity of hair growth and shedding: (a) at inclusion, (b) at 3 months, (c) at 6 months. Ellipse highlights cluster of patients in the placebo group that profited from seasonal hair growth within the first 3 months of treatment. (d) Improvement of anagen rates after 3 and 6 months of treatment (From Hotzenköcherle Trüeb. Inaugural Dissertation, University of Zurich 2015)
The impact of seasonality of hair growth and shedding on clinical trials with hair growth-promoting agents should always be taken into consideration, especially in studies with small numbers of patients and study durations <12 months, since heterogeneity of patient inclusion may be enough to distort clinical efficacy results.
Moreover, seasonal fluctuations in hair growth and shedding may be significant enough to be clinically apparent, specifically in women with patterned hair loss (Fig. 8.2a–c).
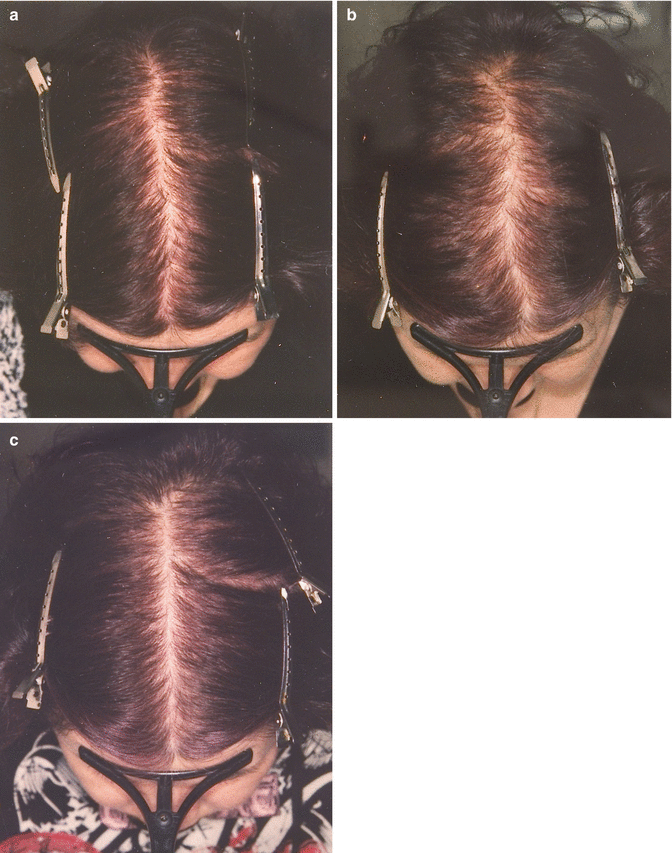

Fig. 8.2
(a–c) Subsequent images taken in (a) January 2007, (b) August 2007, and (c) February 2008 (From Kunz M et al. (2009). Seasonality of hair shedding in healthy women complaining of hair loss. Dermatology 219:105–110)
8.2 Concept of Multitargeted Treatment
Besides an understanding of the pathologic dynamics of hair loss as they relate to the hair growth cycle and integrity of the hair follicle, insight into the multitude of cause-relationships is prerequisite for delivering appropriate patient care. It must be borne in mind that hair loss often does not result from a single cause effect, but from a combination of internal and external factors that all need to be addressed simultaneously in an individualized manner for success, such as smoking and UV radiation, nutritional factors, medications, inflammatory phenomena and scarring, age-related phenomena, and ultimately the problems of comorbidities and multimorbidity in the elderly. The scientific basis for such an approach is given, but there is need for controlled studies to establish increase in efficacy of combination regimens.
8.2.1 Comorbidity
For centuries, physicians propagated the viability of a complex approach in the diagnosis and treatment of disease, while modern medicine, which boasts a wide range of diagnostic methods and variety of therapeutic procedures, stresses specification. This brought up a question: How to wholly evaluate the state of a patient who suffers from a number of diseases simultaneously, where to start from, and which disease(s) requires primary and subsequent treatment? For many years this question stood out unanswered until in 1970 Alvan R. Feinstein came out with the term comorbidity, which has been defined as the presence of one or more additional diseases co-occurring with a primary disease or the effect of such additional diseases, whereby the additional disorder may also be a behavioral or mental disorder.
The effect of comorbid pathologies on clinical implications, diagnosis, prognosis, and therapy of trichologic conditions is polyhedral and patient specific.
The interrelation of disease, age, and drug pathomorphism greatly affects the clinical presentation and progress of the primary nosology, character, and severity of complications; worsens the patient’s life quality; and limits or makes difficult the diagnostic and remedial process. Comorbidity may affect life prognosis and increase the risks of fatality. The presence of comorbidity must be taken into account when selecting the algorithm of diagnosis and treatment plans for any given, including trichologic, disease. Ultimately, the dermatologist participates with the other medical disciplines in the diagnosis and treatment of all types of hair problems as they relate to systemic disease. Selected comorbidities in hair disease are listed in Table 8.2 and illustrated in Fig. 8.3a–u.
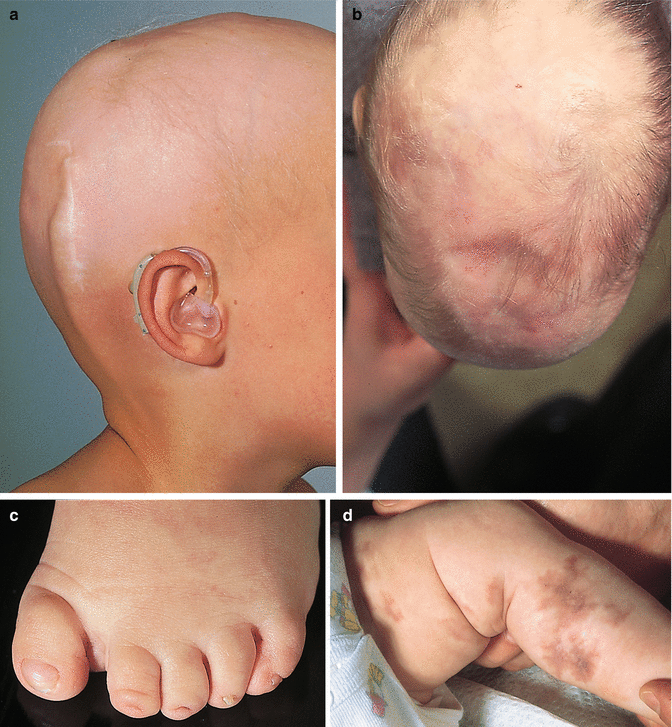
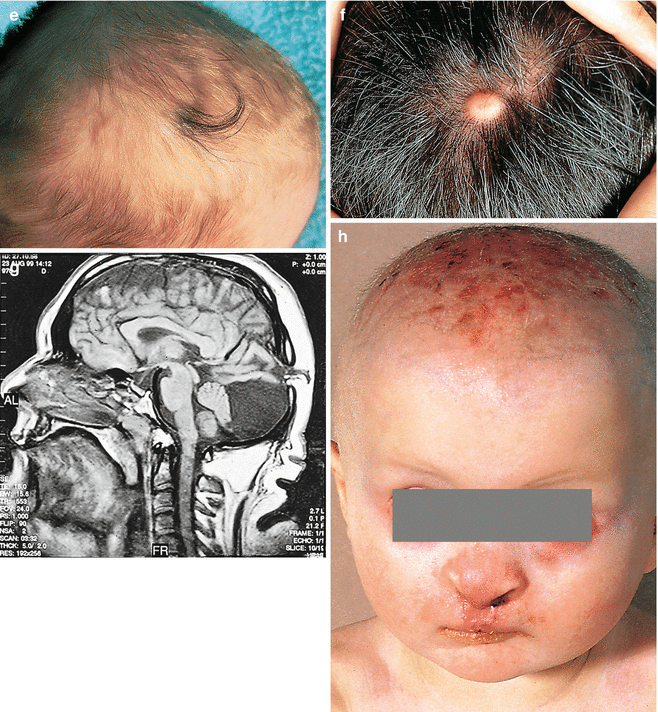
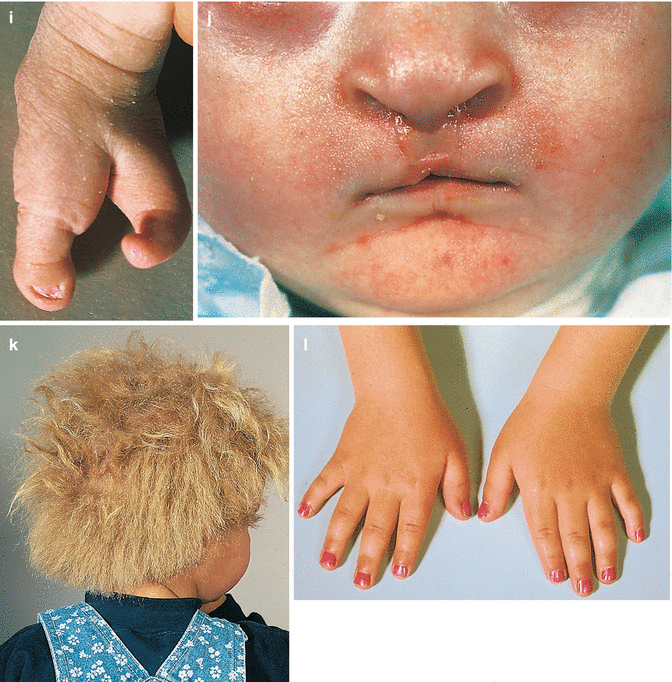
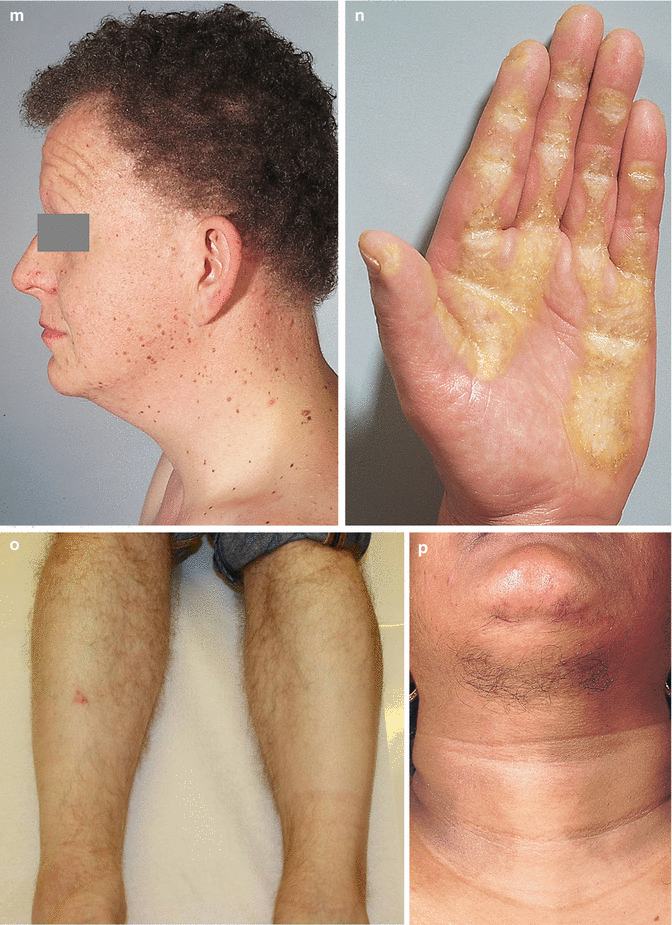
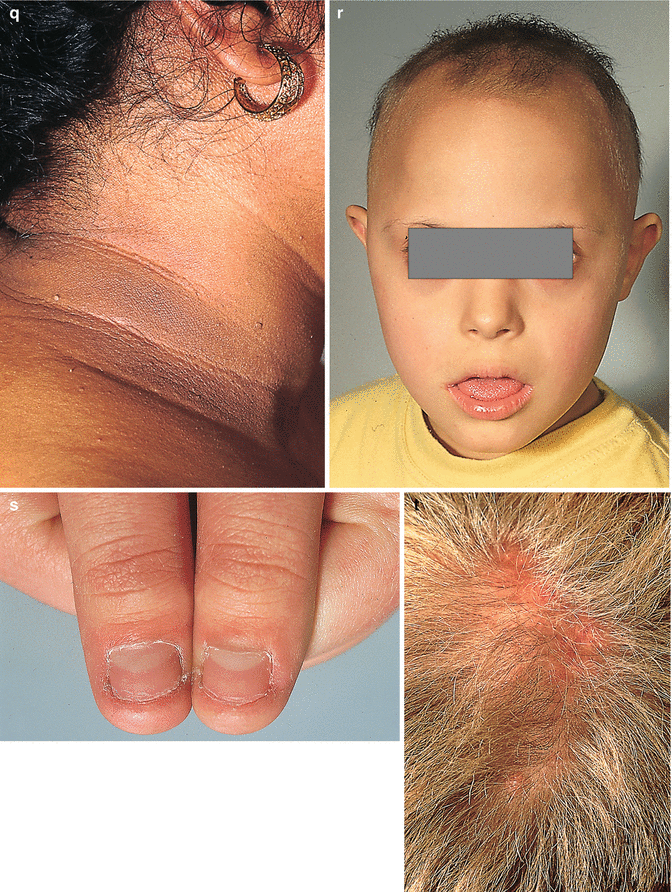
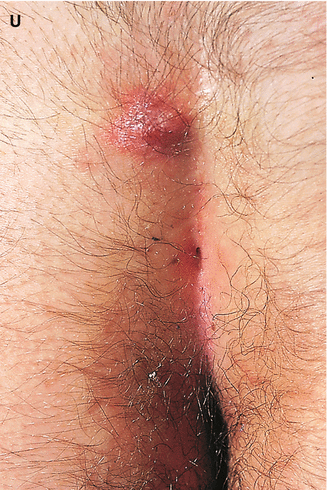
Table 8.2
Comorbidities in hair disease
Condition | Comorbidities |
|---|---|
Congenital disorders | |
Atrichia, hypotrichosis | Mental retardation Epilepsy Immune deficiency Sensorineural deafness Vitamin D-dependent rickets Skeletal anomalies Juvenile macular dystrophy Palmoplantar keratoderma Follicular atrophoderma and basal cell carcinomas (Bazex syndrome) Ectodermal dysplasia |
Alopecia | Holocarboxylase synthetase deficiency (inborn error of biotin metabolism) Biotinidase deficiency (inborn error of biotin metabolism) Acrodermatitis enteropathica (zinc deficiency) Progeria |
Aplasia cutis congenita | Limb anomalies (Adams–Oliver syndrome) Epidermal or sebaceous nevi (SCALP syndrome) Overlying deeper embryologic malformations with hair collar sign Epidermolysis bullosa Fetus papyraceus, placental infarct Teratogens (intrauterine viral infections, carbimazole exposure) Complex malformation syndromes |
Scarring alopecia | Organoid nevi (epidermal, sebaceous) Incontinentia pigmenti (Bloch-Sulzberger syndrome) Epidermolysis bullosa Ichthyosis (alopecia ichthyotica) Ectodermal dysplasia with clefting (AEC and EEC syndrome) Keratosis follicularis (Siemens syndrome) |
Hair shaft anomalies: | |
Pili torti | Menkes syndrome (inborn error of copper metabolism) Sensorineural deafness (Björnstad syndrome) Hypogonadism (Crandall syndrome) |
Twisting dystrophy (pili torti et canaliculi) and corkscrew hairs | Ectodermal dysplasia Clefting (AEC, EEC, and Rapp–Hodgkin syndrome) Ectrodactyly (EEC syndrome) |
Uncombable hair (pili trianguli et canaliculi) | Retinal pigmentary dystrophy Brachydactyly |
Trichorrhexis invaginata | Congenital erythroderma (Netherton syndrome) Atopic disease Ichthyosis linearis circumflexa |
Trichoschisis | Trichothiodystrophy |
Congenital trichorrhexis nodosa | Argininosuccinic aciduria Citrullinemia |
Woolly hair | Palmoplantar keratoderma Arrhythmogenic cardiomyopathy (Naxos disease) Dilated cardiomyopathy (Carvajal syndrome) |
Non-scarring alopecias | |
Male androgenetic alopecia | Insulin resistance Metabolic syndrome Coronary heart disease Obesity Smoking status Anterolateral leg alopecia |
Female androgenetic alopecia | Iron deficiency Hypothyroidism Hyperprolactinemia Polycystic ovary syndrome |
Senescent alopecia | Nutritional deficiency Endocrine disease Drug related Multimorbidity Dementia |
Alopecia areata | Trisomy 21 Atopic disease Autoimmune thyroid disease Pernicious anemia Autoimmune polyendocrinopathy syndrome Celiac disease Iron deficiency Vitamin D deficiency Androgenetic alopecia Trichotillomania |
Trichotillomania | Iron deficiency Onychophagia Trichophagia Trichobezoar Rapunzel syndrome |
Chemotherapy-induced alopecia | Androgenetic alopecia Anti-estrogen therapy Graft-versus-host disease |
Scarring alopecias | |
Lupus erythematosus | Systemic disease (see ACR criteria) |
Frontal fibrosing alopecia | Androgenetic alopecia Autoimmune thyroid disease Lupus erythematosus Lichen planus (mucous membranes, nails) Anterolateral leg-like alopecia |
Folliculitis decalvans | Hypocomplementemia Defective cellular immunity Decreased intracellular killing Hair shaft abnormalities (twisting dystrophy, tufted folliculitis) |
Dissecting cellulitis | Acne conglobata Hidradenitis suppurativa Pilonidal sinus |
All: | |
Psychological comorbidities | Adjustment disorders Personality disorders |






Fig. 8.3
(a–u) Comorbidities in congenital and acquired hair disorders. (a) Congenital atrichia with sensorineural deafness. (b–d) Adams–Oliver syndrome: (b) aplasia cutis congenita, (c) terminal transverse limb defect, and (d) cutis marmorata. (e–g) Cranial dysraphism: (e) hair collar sign, (f) meningocele of the scalp, and (g) MRI of underlying neural tube closure defect. (h–j) EEC syndrome: (h) alopecia with scalp dermatitis, (i) ectrodactyly, and (j) clefting. (k, l) Uncombable hair syndrome with brachydactyly: (k) spun-glass hair and (l) brachydactyly. (m, n) Woolly hair with palmoplantar keratoderma: (m) woolly hair and (n) palmar keratoderma. (o) Anterolateral leg alopecia in androgenetic alopecia. (p, q) Polycystic ovary syndrome: (p) hirsutism and (q) acanthosis nigricans and acrochordons as markers of hyperinsulinemia. (r) Alopecia areata in trisomy 21. (s) Onychophagia (in trichotillomania). (t) Folliculitis decalvans and twisting dystrophy in EEC syndrome. (u) Pilonidal sinus in follicular occlusion syndrome
8.2.2 Value of Nutritional Therapies
Since an important commercial interest lies in the nutritional value of various vitamin and amino acid supplements, an important question that arises is whether increasing the content of an already adequate diet with specific amino acids, vitamins, and/or trace elements may further promote hair growth. Pharmacy aisles and Internet drugstores are full of nutritional supplements promising full, thick, luscious hair for prices that range from suspiciously cheap to dishearteningly exorbitant.
It would appear that unless hair loss is due to a specific nutritional deficiency, there’s only so much that nutritional therapies can do to enhance hair growth and quality. However, there are internal and external factors, such as aging and oxidative stress, that influence hair health to such a degree that nutritional therapy can boost hair that’s suffering from these problems.
Protein is the main component of hair accounting for between 65 and 95 % of the hair by weight. The primary component of the hair fiber is keratin which is made from eighteen amino acids (by alphabetical order: alanine, arginine, aspartic acid, cysteine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophane, tyrosine, and valine). The most abundant of these is cysteine which gives the hair fiber much of its strength through the linking of the sulfur in cysteine molecules of adjacent keratin proteins together in disulfide bonds.
Ingesting keratin does not help hair growth, as the protein cannot be broken down and absorbed (in fact, also rhinoceros horn whose use to cure a variety of ailments is highly praised in the traditional medicine systems in Asia is composed largely of keratin. In other words, there is no evidence to support the plethora of claims about the healing properties of the horns, and one would do just as well chewing on the fingernails or hair). Therefore, constituent amino acids from which the hair follicle can build up the keratin need to be consumed. Cysteine is catabolized in the gastrointestinal tract and blood plasma, while cystine travels safely through the gastrointestinal tract and blood plasma and is promptly reduced to two cysteine molecules upon cell entry. Originally, the role of cystine in the production of wool was investigated in the 1960s, and it was found that enrichment of even what appeared to be a normal diet with the sulfur-containing amino acids cystine and methionine increased wool production in sheep.
The hair follicle exhibits a high rate of metabolism. As a group, B-complex vitamins are important for metabolic functions and therefore required to utilize other nutrients like carbohydrate and amino acids (in alphabetical order): biotin (vitamin H), calcium pantothenate (B5), niacinamide (B3), folic acid, and vitamins B6 (pyridoxal phosphate) and B12 (cobalamin).
When considering which dietary supplements could be used for improving hair growth in humans, cystine in combination with B-complex vitamins or medicinal yeast, a rich natural source of amino acids and B-complex vitamins, was therefore considered. Starting in the early 1990s, studies on the effect of dietary supplements containing cystine, medicinal yeast, and pantothenic acid (CYP-complex) were performed, showing improvements in the trichogram, in hair swelling as a criterion for hair quality, and in the tensile strength of the hair fiber. Eventually, Lengg et al. performed a double-blind, placebo-controlled study in 30 otherwise healthy women suffering from telogen effluvium which demonstrated that a CYP-complex-based dietary supplement increased the anagen hair rate within 6 months of treatment, while placebo did not (Fig. 8.4).
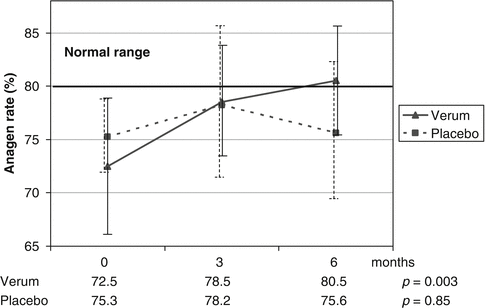

Fig 8.4
Double-blinded, placebo-controlled study in healthy women with hair loss using oral combination of cystine, yeast, and pantothenic acid (CYP-complex): active compound led to statistically significant improvement and normalization of mean anagen hair rates within 6 months of treatment (From Lengg N et al. Dietary supplement increases anagen hair rate in women with telogen effluvium: results of a double-blind placebo-controlled trial. Therapy 2007;4:59–65)
Ultimately, combining with topical minoxidil in the treatment of androgenetic alopecia was speculated to add extra benefit, since it had been shown in whole hair follicle cultures that minoxidil not only increases the incorporation of thymidine as marker of cell division but also leads to increased uptake of cysteine by the hair follicle. This is underlined by the fact that regression analysis in the study performed by Lengg et al. did not show altered treatment efficacy in healthy women with hair loss and concomitant androgenetic alopecia. The proof of concept for superiority of combination treatment with CYP-complex versus minoxidil monotherapy of female androgenetic alopecia was finally presented by Trüeb on the occasion of the 8th World Congress for Hair Research held on May 14–17, 2014 in Jeju Island, Korea: combined therapy resulted in statistically significant higher proportion of patients with normalized percentage of telogen hairs <15 % within 4 months of treatment as compared with minoxidil monotherapy (60 % versus 29 %, p = 0.03).
Interestingly, an experiment performed on C57BL/6 mice that developed hair loss when exposed to cigarette smoke demonstrated that this effect could be prevented by the oral administration of N-acetylcysteine, an analogue and precursor of cysteine and reduced glutathione, as well as cystine, the oxidized form of cysteine. The effect was interpreted as to be possibly related to the glutathione-related detoxification system.
A role of the essential amino acid lysine in hair loss has also been suggested to be important. Double-blind data confirmed the findings of an open study in women with increased hair shedding, where a significant proportion responded to combination treatment with lysine and iron.
While nutritional factors affect the hair directly, one should not forget that they also may affect the skin. In the management of subjects with hair loss, evidence of associated scaling problems should draw the attention to the possibility of deficiency of vitamin A, biotin, zinc, or essential fatty acids.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree