Fig. 15.1
The population of the United States over 65 has been increasing dramatically, a trend that is expected to continue (Source: US Census Data)
The ethnic composition of the United States is expected to change substantially in the coming decades. Between 1900 and 2000, the number of states with at least 10 % of the population that was not Caucasian went from 2 to 26. Between 1980 and 2000, the Hispanic population of the United States more than doubled. By 2000, three US states have majority non-white populations. Globalization of research and innovation will be confronted with the needs of ethnically diverse communities worldwide. Between 1950 and 2000, the United States and other developing nations comprise a diminishing share of the world’s population. These trends will require investigators who are sensitive to and fluent in the needs and cultures of a diverse population.
Gender composition has also changed in the United States and other developed nations (Fig. 15.2). By the middle of the last century, the United States has gone from a majority male to a majority female nation. The sex ratio of males to females has declined every decade from 1910 to 1980. This ratio increases with increasing age. Between 1900 and 2000, the number of states with a majority female population has increased fourfold, from 11 states to 44. Over the same century, the nation has gone from primarily agrarian to primarily urban and suburban in population.
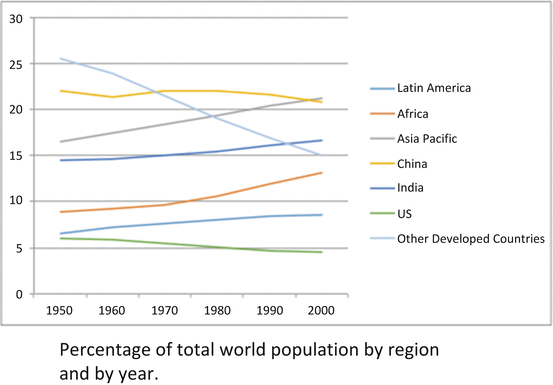

Fig. 15.2
The percentage of the world population represented by the United States and Westernized nations is declining. This is the opposite of population trends in Africa, Latin America, and Asia
From a public health perspective, older individuals will consume more healthcare services. Around 80 % of individuals aged 65 and older suffer from at least one chronic health condition, and 50 % suffer from at least two. Diabetes affects nearly 19 % of this population. Diabetes is associated with a number of cutaneous manifestations. A number of skin diseases are associated with the elderly. These can range from disorders of cell proliferation such as skin cancer, to diseases of the skin barrier, the immune response, diseases associated with metabolic and nutritional changes. The normal process of aging can also affect the skin with decreased elasticity, thinning of the dermis, fragility of the cutaneous vasculature, graying of the hair, and nail dystrophy. Infections are common in the elderly population, including onychomycosis, bacterial infections, and intertrigo. Viral infections such as herpes zoster can have severe manifestations in the elderly. Hospitalized or bedridden aged are at greater risk for pressure ulcers and institutional infestations such as pediculosis and scabies. The elderly are on multiple medications and are at greater risk for drug reactions, many of which affect the skin. Autoimmune bullous dermatoses such as bullous pemphigoid tend to occur in the elderly. Paraneoplastic autoimmune bullous dermatoses such as paraneoplastic pemphigus are more common in older individuals. Itch is very common in the elderly and represents a major cause of morbidity and reduced quality of life.
The demand for cosmetic dermatologic treatments for androgenetic alopecia, hirsutism associated with aging, rhytids, and therapies for improving skin texture and dyschromia will grow. Benign neoplasms such as acrochordons and seborrheic keratosis and lentigines tend to proliferate in the elderly.
Nanotechnology is the study of materials and devices 100 nm in size and smaller. It capitalizes on the unique properties of matter engineered at this size scale to create entities with novel characteristics. Nanotechnology-based materials have been developed for consumer, military, and industrial use. Dermatology is one of the fastest growing beneficiaries of nanotechnology over the past decade. Some of the top holders of patents in nanotechnology include dermatology and cosmetics companies. Nanotechnology intersects with biology, materials science, chemistry, physics, quantum mechanics, pharmacology, molecular biology, bioinformatics, genomics, and proteomics to revolutionize the diagnosis and management of skin disease and for the maintenance of skin health. Nanomaterials have been created for photoprotection, and for enhancing the appearance of the skin, hair, and nails. They are being developed for the targeted treatment of a number of skin disorders, from the use of gold nanoshells for treating acne and melanoma, to thermosensitive nanopolymer gels for the prevention of the transmission of HIV, to liposomal nanoparticles for the delivery of retinoids and ceramides [1, 2]. While some advances in dermatology therapeutics may have reached a plateau, with “low hanging fruit” already harvested, nanotechnology is expected to broaden the horizon of discovery significantly, and greatly add to the future of skin health.
These trends in demographics and discovery have generated both an urgent demand for new skin treatments and a wide pipeline of products and technologies. As discussed in previous chapters, one of the biggest bottlenecks for bringing basic science ideas from the bench to the bedside is human subject clinical trials. The clinical trials phase is the most expensive and potentially time consuming aspect of the approval process. Delays in this phase can add substantially to cost overruns. They can also cost competitive advantage and shorten patent exclusivity. Protocols have become more complex and costly in recent years demanding even more of sophistication and training investigators and investigative site staff.
These trends make for a relatively positive career outlook in investigative dermatology. The need for clinical investigators is expected to grow by 13 % over the next decade compared to 9 % for other careers. Almost 75 % of investigators receive no formal training. As protocols and investigational products become more complex, expectations are that formal training courses and requirements will increase. The number of formal courses and training opportunities has increased. There are courses in good clinical practices as well as formal fellowships in clinical research and clinician scientist and medical scientist training programs.
Currently, there is a shortage of investigators [2–6]. It takes years to train adept clinicians to be qualified dermatology investigators. In addition to the long years of training for a medical education and residency, there is the training in the scientific method and in good clinical practices (GCPs) for the safe and objective conduct of clinical trials involving human subjects.
15.1 Clinical Trials Enhance Your Practice
By conducting clinical trials, you may become a recognized expert in your locale. Patients and colleagues can learn about studies in your practice, which may be a useful source of referrals. Speaking engagements at local, regional, and national meetings can be forums for heightening your practice’s visibility. This increased visibility can lead to a virtuous cycle of increased referrals for patient care as well as potential participations in current and future studies at your site. If you become a highly sought-after investigator, you may be offered co-authorship on key publications related to your research.
Clinical trials give you a break from the routine of patient care. Conducting research trials may make you a better clinician [7]. They allow you to approach medicine and a disease from a future-oriented, scientific perspective. They require you to review and understand pathophysiology, pharmacology, epidemiology, biostatistics, and study design. They require you to understand currently available alternatives to management of a particular disease. They expose you to the state-of-the art of a particular skin condition. Research trials often involve training which may augment or hone your clinical skills in diagnosing or evaluating a particular dermatologic disease. For many studies, you will be required to conduct complete physical examinations, review a variety of laboratory data—electrocardiograms, imaging studies—and review medical records including hospital records. This will keep your overall medical skills sharp and your awareness of a broader array of medical problems up to date. The extended contact involved with study participants with a particular disease may give you a better understanding of quality-of-life measures and other impacts of a disease on a subject and his/her contacts.
As you conduct studies, you will enhance your observation skills, and objectivity. You may take advantage of this by being a better diagnostician, or by beginning to develop and formulate hypotheses to conduct your own studies. In fact, many dermatologic therapies have resulted from such observation being put to use in clinical trials. Examples include observations of hair growth and lengthening associated with bimatoprost—originally used to treat open angle glaucoma—and minoxidil—a vasodilator developed for the treatment of hypertension; diminution of unwanted hair in subjects using eflornithine—originally developed for trypanosomiasis—and decreased flushing and telangiectasia in rosacea by brimonidine.
You will also be giving back. By offering novel therapies, and by providing care for those who can’t afford it, you will be an active participant in optimizing care for your community. You will also contribute to the advancement of medical and scientific knowledge.
15.2 Clinical Trials Offer Leadership Opportunities
Due to the short supply of physician scientists (currently less than 5 % of the physician workforce), the job market in this field is excellent. They are highly sought-after to become the future leaders of medicine and dentistry. Physician scientists hold leadership positions wherever they are: in academia, government, and private industry (including pharmaceutical, biotech, and venture capital companies). There are a number of industries interested in career investigative dermatologists. These include: pharmaceutical, biopharmaceutical, biologics, contract research organization, trial management organization, site management organization, medical research facility, university, consulting company, and device manufacturer. If you work for the government, you could work at the FDA, CDER, or CBHR. Specific jobs in industry include: clinical research physician, medical communications officer, manager clinical programs, director clinical trials office, regulatory affairs director, drug safety liaison, and epidemiologist. You could work as a principal investigator, a co-investigator, study manager, medical monitor, medical director, field physician, medical writer, chief medical officer, insurance company case reviewer, laboratory director, or product vigilance director. Depending upon your knowledge of statistics or information technology, you could be data coordinator, a biostatistician, a study designer, a clinical IT specialist, data analyst, data manager, or project manager. You could also be responsible for quality assurance as a QA/QC auditor, a technical writer, a regulatory specialist, or a QA/QC manager.
15.3 Challenges of Clinical Trials
The challenges of clinical research operations are rising costs, the majority of which go to study personnel, with salaries consuming nearly 60 % of research grants. In addition to study staff, legal fees and compliance review costs continue to rise. Malpractice premiums, training costs, and overhead are increased as trials become more complex, and study requirements more onerous for study participants and study sites [8–11].
The costs of maintaining a medical practice are also growing, for many of the same reasons. These are rising overhead, declining reimbursement, and ever greater regulatory burdens.
If budgeted and run efficiently, clinical trials can generate modest income to offset losses or at least diversify practice income. Any additional income can be reinvested in the practice, or used as a cushion to sustain the practice during ebbs in revenue.
It’s harder for medical practices to recruit physicians, especially in rural areas. Pressures are discouraging doctors from continuing to practice. These include diminished income, autonomy, and increased overhead. Research can help offset some of these costs. But profit margins are suitable for most dermatology practices. Phase II trials are slightly better compensated than Phase I, III, or IV.
15.4 Training Options/Career Paths
There are a variety of formal and informal ways of developing the skills necessary to become a competent investigator. One of the easiest is through networking. You can seek out dermatologists in your area who are conducting clinical trials. Offer to help by asking to be a subinvestigator. This will allow you to review the protocol, get appropriate protocol-specific training, see study subjects, and work closely with co-investigators, the PI, medical monitors, clinical research coordinators, and sponsors. If you have the opportunity to attend an investigator meeting, take it. Investigator meetings are good ways to meet new people and to network. They also provide detailed information on the study in detailed study packets combined with online training content. You will get presentations from noted experts including: scientific background on the investigational product or device by a bench research scientist, perhaps even the discoverer of the patent. The sponsor may go over the pharmacoeconomics of a particular agent. A medical monitor will review clinical data from earlier trials. You will see raw data from PK studies are reviewed. In-house MRAs or CRAs will cover topics related to the protocol such as inclusion and exclusion criteria, a study worksheet, case report forms, data collection, subject recruitment and retention, labs, investigational product dispensing and collecting, data entry, and adverse events. You will get presentations from laboratory personnel who discuss expected lab findings and anomalies. You will hear from regulatory personnel on dealings with regulatory agencies such as the FDA. You will also learn a lot from the discussions that ensue. For example, some attendees may question the study design, or details of the protocol. Obvious and subtle omissions or errors will be debated, sometime in great detail. You will come away from almost any investigator meeting with a deeper understanding of the clinical research process and a number of contacts to work with in the future.
There are standard web-based training courses. One of the best is the Collaborative Institutional Training Initiative (CITI) program. Recommended modules for investigators doing clinical trials include: good clinical practices for clinical trials involving drugs and devices; overview of new drug development; overview of international conference on harmonization; conducting investigator-initiated studies according to FDA regulations; investigator obligations in FDA-regulated clinical research; managing investigational agents according to good clinical practices; overview of FDA regulations for medical devices; informed consent; detecting and evaluating adverse events; reporting serious adverse events; audits and inspections of clinical trials; humanitarian use devices; and monitoring of clinical trials by industry sponsors. The National Cancer Institute (NCI) offers training on cancer clinical trials at www.cancer.gov/clinicaltrials/conducting/clinicaltrialscourse




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






