, Teresa S. Wright2, Crystal Y. Pourciau3 and Bruce R. Smoller4
(1)
Department of Pathology & Immunology, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA
(2)
Departments of Dermatology and Pediatrics, University of Tennessee Health Science Center, Memphis, TN, USA
(3)
Departments of Dermatology and Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA
(4)
Department of Pathology and Laboratory Medicine, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA
15.1 Acrodermatitis Enteropathica
15.1.1 Clinical Features
Zinc deficiency may be acquired or inherited. The inherited form is termed “acrodermatitis enteropathica” and affects 1 in 500,000 infants [1]. Acrodermatitis enteropathica most commonly presents within weeks of transitioning from breast milk to formula milk secondary to the decreased bioavailability of zinc in manufactured, dairy-derived milk product [1]. Acquired forms of disease may be secondary to inadequate intake (as seen in diets with limited animal-derived food), excessive losses (as seen in some malabsorption diseases, such as celiac disease, ulcerative colitis, and cystic fibrosis), and increased demand (as seen in pregnancy, lactation, and prematurity) [2].
The classic mucocutaneous feature of zinc deficiency is an eruption of erosive and desquamating, scaly eczematous plaques at the distal extremities, anogenital and peri-orificial skin (Figs. 15.1 and 15.2). Alopecia, angular cheilitis, and paronychia can be seen as well. Diarrhea is also a frequent feature. In the majority of cases, symptoms resolve with treatment of the predisposing condition and zinc supplementation [2]. Seventy percent of patients have improvement in zinc levels within 6 months of initiation of zinc supplementation. Unfortunately, patients with acrodermatitis enteropathica require life-long zinc supplementation.
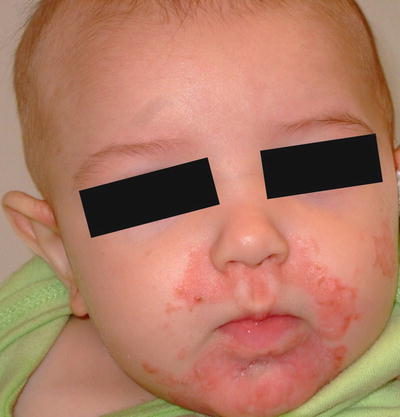
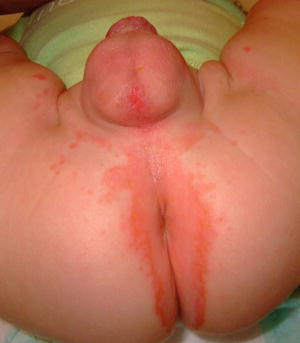

Fig. 15.1
Peri-oral erythematous scaly plaques are seen in an infant with acrodermatitis enteropathica

Fig. 15.2
Peri-anal and genital erythematous, scaly papules and plaques with superficial erosions in the same infant with acrodermatitis enteropathica
15.1.2 Histology
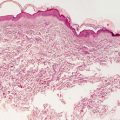
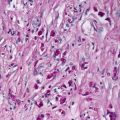
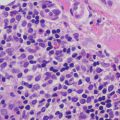
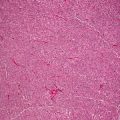
Histologic changes in acrodermatitis enteropathica include confluent parakeratosis, psoriasiform epidermal hyperplasia, marked diminution or total loss of the granular layer, marked spongiosis, and pronounced epidermal pallor limited to the upper one-half to one-third of the epidermis [3–5] (Figs. 15.3 and 15.4). Ballooning degeneration of the keratinocytes contributes to the pale staining within the epidermis. There are neutrophilic abscesses within the stratum corneum and upper portions of the epidermis. Within the dermis, the most common findings are a superficial perivascular, predominantly lymphoid infiltrate, occasionally with scattered eosinophils or neutrophils. Focal acantholysis has been described in lesions of acrodermatitis enteropathica [6]. Bullous variants and the presence of interface dermatitis have also been reported [7].
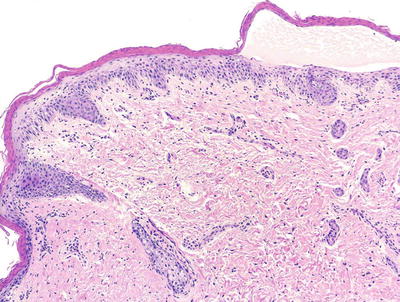
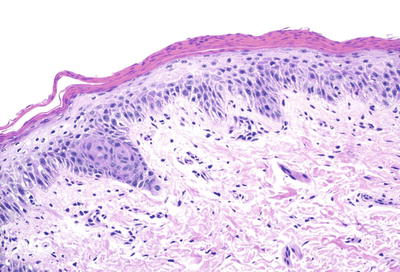

Fig. 15.3
Histologic findings in acrodermatitis enteropathica include pallor of keratinocytes in the upper portion of the epidermis, sometimes resulting in superficial blister formation. Minimal dermal inflammation is seen in most cases

Fig. 15.4
Thick parakeratosis overlies pale staining epidermis with focal hypogranulosis in acrodermatitis enteropathica
The histologic changes of acrodermatitis enteropathica are identical to those seen in necrolytic migratory erythema and necrolytic acral erythema. The former is associated with glucagonoma, and the latter is associated with hepatitis. Both diseases are rarely encountered in children, and are easily differentiated based upon clinical presentation. Another entity that must be considered in the differential diagnosis is psoriasis. The epidermal pallor that is characteristic of acrodermatitis enteropathica is not seen in psoriasis; however, early lesions of acrodermatitis enteropathica do not demonstrate this finding. The earliest changes may be virtually indistinguishable from psoriasis, and close clinical examination and follow-up may be the only way to make the distinction. In some situations, spongiosis may be the most significant histologic alteration in early lesions, and the differential diagnosis includes other causes for spongiotic dermatitis, such as atopic dermatitis, contact dermatitis, an id reaction, and nummular eczema. Again, clinical correlation is the most reliable way to make the distinction. As the lesions develop, the distinction becomes clearer based upon the specific and often dramatic epidermal pallor .
15.1.3 Pathogenesis
Acrodermatitis enteropathica is caused by an autosomal recessive defect in the zinc transporter SLC39A4 (Zip4 transporter) [8, 9]. SLC39A4 is expressed at the apical surface of mature enterocytes of the intestinal villi [10, 11]. The function of SLC39A4 is to transport zinc to enterocytes in the small intestine [12]. Thus far, 41 mutations in SLC39A4 have been identified in patients with acrodermatitis enteropathica. A number of mutations occur in the N-terminal domain of the gene. These mutations appear to block the proteolytic cleavage of the protein product, leading to impaired protein function. Interestingly, acrodermatitis enteropathica with hereditary zinc deficiency due to mutations in SLC39A4 also exists in animals, including Holstein-Friesian cows, northern-breed dogs, and goats [13–15]. Thus, SLC39A4-associated zinc deficiency is not restricted to humans.
A condition called transient neonatal zinc deficiency is due to zinc deficiency that arises from inadequate supplementation in total parenteral nutrition or low breast milk levels. The defect is due to mutations in the SLC30A2 gene that causes decreased secretion of zinc into the mother’s breast milk [10, 16, 17]. As a consequence, the breast-fed child develops a zinc deficiency. This condition can be rapidly corrected with zinc supplementation .
15.2 Pellagra
15.2.1 Clinical Features
Pellagra is niacin/vitamin B3 deficiency. Vitamin B3 is a water-soluble vitamin found in whole grains, meat, dairy, nuts, and beans. Pellagra is characterized by a triad of dermatitis, diarrhea, and dementia. It was previously most commonly seen in populations with diets primarily consisting of corn [1]. Following initiation of food fortification efforts, more recent cases of disease are associated with dietary restrictions, medical conditions, such as malabsorption disorders, alcoholism, carcinoid syndrome and HIV disease, as well as certain medications, including isoniazid, pyrazinamide, 6-mercaptopurine, 5-fluorouracil, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol [1].
Patients present with eczematous patches around the neck (Casal’s necklace) with progression to a well-demarcated, photodistributed erythematous eruption, which may be exudative, vesicular, or desquamative [1]. Lesions evolve to become more hyperkeratotic and brown in color over time. Initial neurologic symptoms may be minimal, including headache and irritability, with progression to encephalitis, neuritis, and myelitis in more severe disease. Patients may improve with correction of the underlying disorder or supraphysiologic dosing of niacin.
15.2.2 Histology
Histologic features of pellagra are essentially indistinguishable from those seen in acrodermatitis enteropathica [18–20]. Confluent parakeratosis overlies psoriasiform epidermal hyperplasia with marked epidermal pallor in the superficial portion of the epidermis in well-developed lesions (Figs. 15.5 and 15.6). Ballooning degeneration of keratinocytes is present. Spongiosis is present in early lesions, but less common in later stages of development. Vascular ectasia and a mild perivascular lymphocytic infiltrate is present in the papillary dermis.
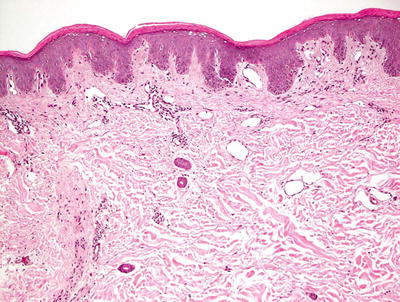
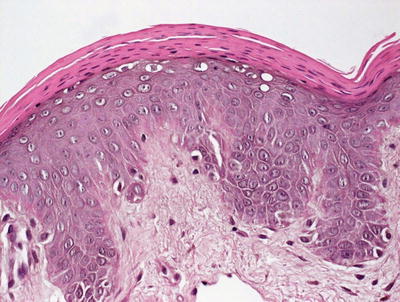

Fig. 15.5
Pellagra is characterized by confluent parakeratosis overlying an epidermis that contains vacuolization and pallor in its upper layers. Psoriasiform epidermal hyperplasia is seen in some cases

Fig. 15.6
Thick adherent parakeratosis and vacuolar degeneration of the superficial layers of the epidermis are common findings in biopsies from patients with pellagra
The differential diagnosis includes other nutritional disorders that display cutaneous manifestations, including necrolytic acral erythema, necrolytic migratory erythema, and acrodermatitis enteropathica, each of which is distinguished based upon clinical factors. Psoriasis and spongiotic dermatoses also enter the differential diagnosis, depending upon the stage of lesion biopsied, but can usually be distinguished based upon clinical presentation .
15.2.3 Pathogenesis
Niacin is an essential component in the synthesis of nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) [1, 21, 22]. NAD and NADP are important coenzymes in catabolic and anabolic reactions. Numerous enzymes require NAD and NADP to act as hydrogen ion donors and acceptors in the catabolism of carbohydrates, fat, and proteins, as well as in anabolic reactions in the synthesis of fatty acids and cholesterol [23]. One of the cutaneous manifestations of pellagra is photosensitivity. Although the mechanism of photosensitivity is not known, there are several proposed theories, including cutaneous deficiency in urocanic acid, accumulation of kynurenic acid, NAD/NADP deficiency, and abnormal metabolism of porphyrin [22, 24, 25].
15.3 Kwashiorkor
15.3.1 Clinical Features
Kwashiorkor is protein deficiency in the setting of appropriate calorie ingestion. Children at greatest risk for disease are those in developing countries recently weaned from breast milk [1]. In developed countries, kwashiorkor is the most common form of malnutrition in hospitalized children, and it is most often seen in the setting of prematurity, chronic malabsorption conditions, and restrictive diets.
Patients present with generalized edema, “flaky paint dermatitis ,” hair loss with lightened pigmentation, and “flag sign” (alternating bands of normal pigmentation and hypopigmentation in the hairs) on microscopy. Body weight is typically >60 % of age matched controls [1]. Neurologic sequelae of irritability and lethargy can be seen in more advanced disease. Severely affected children are at increased risk for sepsis and circulatory collapse secondary to low protein production.
15.3.2 Histology
The histologic findings in kwashiorkor are not specific, and essentially the same as those found in acrodermatitis enteropathica and other nutritional deficiencies [26–28]. Similar changes can be seen on mucosal surfaces, especially the tongue [28]. Extreme malnutrition in kwashiorkor can also lead to decreased melanin production in hair follicles, resulting in spotty, focal hypopigmentation of hair [26, 29, 30]. The histologic differential diagnosis includes other nutritional deficiencies as described elsewhere in this chapter.
15.3.3 Pathogenesis
A number of nutrients have been suggested to play a role in kwashiorkor, including proteins, sulfur amino acids, zinc, and essential fatty acids [31–33]. Children with kwashiorkor have been shown to have different amino acid and collagen status in their skin as compared with normal healthy children [34, 35]. In this condition, there are higher concentrations of markers of oxidative stress and lower levels of antioxidants in the blood, indicating excessive oxidative stress [31, 36]. Kwashiorkor may be a result of an imbalance between the production and removal of free radicals [36–38]. Excess free radicals, as well as reduced levels of glutathione, have been demonstrated in kwashiorkor [39, 40].
15.4 Scurvy
15.4.1 Clinical Features
Routine screening for vitamin C deficiency in the general population has demonstrated a prevalence of 13–30 % for vitamin C depletion, and 5–17 % for frank vitamin C deficiency [1]. Affected persons range in age from neonates to the elderly. Decreased levels may be secondary to avoidance diets, gastrointestinal illness with malabsorption, and psychiatric illnesses, such as substance abuse. Frank deficiency typically is not seen until patients have had almost no intake for 2–3 months. Möller-Barlow disease is a disease of vitamin C deficiency in infants with pain and pseudoimmobilization of the hips and knees, frequently secondary to subperiosteal hemorrhage [1].
Physical exam is characterized by follicular hyperkeratosis with progression to perifollicular hemorrhage, corkscrew hairs, and generalized ecchymoses. Patients may also complain of poor wound healing. Anemia and gingivitis with subsequent tooth loss can be seen. Deficiency is corrected with vitamin C supplementation, but death can occur in untreated patients secondary to bleeding complications.
15.4.2 Histology
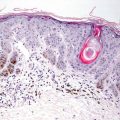
Histologic changes of scurvy include hyperkeratosis overlying the acrotrichia, resulting in plugged hair follicles [41, 42]. Follicular epithelium within the dermis may appear hyperconvoluted, and demonstrate dilated medullary cavities. In the dermis surrounding hair follicles, there is a mild to moderate infiltrate of lymphocytes. Perifollicular hemorrhage is also an expected finding with hemosiderin present in more chronic lesions.
The histologic differential diagnosis includes pigmented purpuric eruption and leukocytoclastic vasculitis [42, 43]. There is no transmural infiltrate of neutrophils, or fibrinoid necrosis of vessel walls in scurvy, unlike in leukocytoclastic vasculitis. Pigmented purpuric eruptions are not expected to have follicular dilatation or overlying hyperkeratosis .
15.4.3 Pathogenesis
Scurvy is due to a deficiency in vitamin C (ascorbic acid). Vitamin C has many important cellular functions, including its role as an antioxidant, and its function in hydroxylation of collagen in connective tissues, biosynthesis of carnitine and norepinephrine biosynthesis, and tyrosine metabolism [44–46]. Scurvy is more commonly encountered in adults, but it can also be seen in children [47, 48]. Groups at risk for scurvy include infants who are fed evaporated or boiled milk, in which vitamin C is destroyed by heat, and children with dietary restrictions stemming from psychiatric or developmental disorders [47, 49]. Scurvy can also mimic child abuse or parental neglect. Scurvy is easily diagnosed by testing for serum levels of vitamin C (a level of less than 11 micromol/L suggests scurvy) [48]. The disorder can be corrected rapidly with vitamin C supplementation .
15.5 Vitamin A Deficiency
15.5.1 Clinical Features
Vitamin A deficiency is seen most commonly in patients with malabsorption secondary to medical or surgical interventions and with liver disease [1]. Patients with vitamin A deficiency usually present with vision changes, including poor adaptation to light, corneal xerosis, ulceration, and keratomalacia, with potential for progression to blindness [1]. Mucocutaneous features of disease include xerosis with severe scaling and fissuring, as well as perifollicular hyperkeratosis (phrynoderma), which is highly characteristic but not specific of disease. In phrynoderma, patients present with filiform papules that evolve into larger papules and plaques with hyperkeratotic horny centers. These lesions present along the extensor surface of the extremities, shoulders, and buttocks. In more severe disease, lesions may generalize and involve the entire body [50]. Patients often improve with better overall nutrition.
15.5.2 Histology
Histologic findings in perifollicular hyperkeratosis/phrynoderma are subtle and can be easily overlooked. Biopsies demonstrate hyperkeratosis, perhaps more prominent overlying the outflow tracts of hair follicles. There is subtle follicular dilatation and minimal lymphocytic infiltrate [50, 51]. A perforating folliculitis is also present in some cases [52].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree