(1)
Obesity Institute, Geisinger Medical Center, Southold, NY, USA
Abstract
The gastrointestinal anatomic changes associated with bariatric surgical procedures result in altered absorptive capability for minerals such as Iron, Zinc, and Copper, which are normally absorbed in the duodenum. Mineral deficiencies are now being recognized with increasing frequency in patients following bariatric surgery. For prevention and management of deficiency, specialized nutritional follow-up by individuals familiar with the assessment of mineral nutrition and the recognition of deficiency states is an essential component of multidisciplinary management. Improved protocols for nutritional follow-up are essential for improving the value of bariatric surgery and expanding patient access to this treatment.
Iron
The importance of iron nutrition is fairly well known to bariatric centers because iron deficiency is one of the more common nutritional complications of bariatric surgery. Iron is a critical element in cellular function. It has a major role in oxygen transport as an oxygen carrier in the hemoglobin molecule. Heme iron is also bound to muscle myoglobin. Iron is involved as a component of the cytochrome enzyme system in mitochondrial electron transport. Iron is available in diet in two forms, molecular and heme iron. Meat is a major source of heme iron in the diet and 2/3 of the body iron store of 4–5 g in well-nourished adults is derived from heme iron.
Molecular iron enters the stomach in the oxidized (Fe3+) form. Gastric acidity and ascorbic acid facilitate the solubilization of elemental iron. Absorption of iron takes place in duodenal and proximal jejunal mucosal cells. These absorptive cells then release iron to the circulation bound to transferrin. Heme iron is also absorbed in the duodenum where pancreatic enzymes free the heme moiety from dietary hemoglobin and myoglobin. The recommended daily allowance for iron is 8–18 mg per day. Iron stores are mainly in liver, spleen, and bone marrow. There is no excretory pathway for iron, with daily loss coming only from loss of epithelial cells from skin, urinary epithelium, gastrointestinal mucosa, and loss of blood.
Factors that contribute to iron deficiency include an increase in iron demand (pregnancy and growth), increased loss of iron (bleeding, menses), and diminished dietary intake (dietary deficiency or malabsorption). The stages in the development of iron deficiency and the diagnostic tests are summarized in Table 8.1. In the initial phase of progression to deficiency, there is a period of negative iron balance where the daily demands for iron and/or losses of iron exceed the iron available in diet. During this period, iron stores make up the deficit and iron homeostasis is maintained until stores are depleted. During this period, the only hint of developing problems with iron is a falling ferritin level. Under most conditions, the serum ferritin level correlates with iron stores and when the level falls to ≤15, iron stores are depleted and the deficiency state begins. When stores are depleted, the serum iron level will begin to fall reflecting deficiency. Hemoglobin synthesis is preserved while iron levels remain in the normal range. Once the transferrin saturation falls to 20 %, hemoglobin synthesis becomes impaired and anemia develops.
Table 8.1
Laboratory values at various stages in the development of iron deficiency
Laboratory assessment of iron nutrition | ||||
|---|---|---|---|---|
Iron status | Normal | Depletion, no deficiency | Deficiency, early anemia | Severe deficiency |
Marrow iron stores | Normal | Reduced | Absent | Absent |
Plasma ferritin, level (mcg/l) | 60–140 | <25 | <15 | <10 |
Hemoglobin level (g/dl) | Normal | Normal | 9–12 | 6–7 |
Transferrin IBC (mcg/l) | 300–360 | 330–360 | 390 | 410 |
Transferrin saturation (mcg/dl) | 20–50 | 30 | <15 | <15 |
Mean corpuscular volume | 80–100 | 80–100 | <80 | <65 |
The prevalence for iron deficiency among candidates for bariatric surgery on the basis of abnormal blood levels is 1–15 %, with a large majority in females [1–5]. Recent evidence suggests that negative iron balance is an inevitable consequence of bariatric surgical procedures and that the progression to iron deficiency is a major risk, but responsive to good surveillance and supplementation. Studies using test meals of labeled inorganic and heme iron show that absorption of both is significantly impaired 12 months following gastric bypass and sleeve gastrectomy in comparison to before surgery (Fig. 8.1) [6]. Mechanisms for the diminished absorption relate to loss of gastric acidity, bypass of the duodenum, and delay in the contact of the food bolus with pancreatic and biliary secretions [7]. In addition to major limitations in absorption, diet reviews indicate that daily intake of iron in the diet of postoperative bariatric surgery patients is well below the recommended dietary allowance [8].
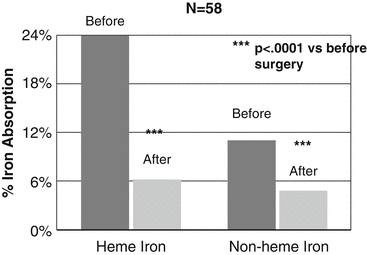

Fig. 8.1
Iron absorption measured after a test meal containing labeled heme and inorganic iron. The test meal was given before and 12 months after gastric bypass and sleeve gastrectomy. Adapted from Ruz M, Carrasco F, Rojas P, Codosceo J, Inostroza J, Basi-fer K, et al. Heme- and Nonheme-iron Absorption and iron Status 12 Months After Sleeve Gastrectomy and Roux-en-Y Gastric Bypass in Morbidly Obese Women. Am J Clin Nut 2012;96:810–817 [6]
This evidence suggests that depletion of iron stores and deficiency is inevitable after bariatric surgery without judicious follow-up and supplementation. Iron depletion and deficiency anemia are common after all types of bariatric surgery. The prevalence was summarized in a recent review of retrospective reports, which indicated a wide-ranging prevalence. After purely restrictive procedures, rates of 0 % at 1 year to 32 % at 4 years are reported. Following gastric bypass, rates of 13–52 % are reported with higher rates occurring with longer follow-up. After malabsorptive procedures, rates of 20–45 % are reported [9]. Variation in reported rates is a reflection of differing care plans for iron supplementation and variations in patient compliance with supplementation. One report of a 0 % incidence of iron deficiency in a cohort of 589 patients followed over 3 years following biliopancreatic diversion with duodenal switch indicates that good follow-up and supplementation can potentially control this condition. In addition, this report raises questions about the nutritional significance of partial preservation of the duodenum in the duodenal switch procedure [10].
The evidence to date indicates that iron nutrition can be managed in bariatric surgery patients with careful follow-up, supplementation, and surveillance for iron depletion. Several studies of inorganic iron tolerance with blood levels following an oral dose of elemental iron indicate that absorption takes place following bariatric surgery [11, 12]. In the majority of postoperative patients, iron nutrition can be managed with oral iron therapy. Multiple oral preparations are available from simple iron salts to more complex preparations designed for sustained release. For treatment of deficiency, up to 300 mg of elemental iron may be given daily, usually in divided doses of 50–65 mg of elemental iron through the day (Table 8.2).
Table 8.2
The commonly used oral iron supplements and the amount of elemental iron available
Preparation | Elemental iron (mg) |
|---|---|
Ferrous gluconate (325 mg) | 39 |
Ferrous sulfate (325 mg) | 65 |
Ferrous fumarate (325 mg) | 107 |
In patients with limited gastric retention capacity, iron solutions are a consideration. In the normal individual, such therapeutic doses of iron should allow for absorption of up to 50 mg per day. The rate of response in the post-bariatric surgery patient is a function of how much absorption takes place and will require close monitoring of dose and response. The goal of treatment is not only to resolve anemia, rather to partially restore at least some part of the iron stores. Continued treatment after resolution of anemia and monitoring serum ferritin should accomplish this. There is some evidence that the response to oral iron may be augmented by the simultaneous administration with ascorbic acid to enhance absorption [12]. Because of the limited human capability for iron excretion, injudicious treatment or even supplementation can lead to iron overload.
Unfortunately, patient compliance with oral iron treatment or supplementation may be a challenge for the bariatric center because of the common occurrence of unpleasant gastrointestinal symptoms (nausea, vomiting, abdominal pain, and constipation) in association with iron therapy. This mandates close communication with patients, possible changes in iron preparation, and regular checks on patient compliance. Failure of oral therapy is an indication for referral for parenteral iron replacement, which is an increasingly common occurrence after bariatric surgery, especially for women of childbearing age [13]. It is evident that skilled nutritional care, patient teaching, and careful follow-up are essential in the preservation of quality of life in regard to iron nutrition.
Zinc
Zinc is the second most common trace element in the body. Total body zinc amounts average 1.5–2.5 g, slightly less than total body iron. The majority of total body zinc (60 %) is in low turnover pools in muscle and bone. Zinc is essential for normal cellular metabolic activity as it is a component of 250 important proteins including Angiotensin Converting Enzyme, Alkaline Phosphatase, Carbonic Anhydrase, and DNA Polymerase. Its importance lies in stabilization of protein and DNA structure, protection from free radical damage, growth and development, and wound healing. It also has a role in cell division and apoptosis. The major site for gastrointestinal absorption is the duodenum and proximal jejunum, with absorption regulated by zinc nutritional status. Pancreatic enzymes are necessary for the release of dietary zinc. Dietary sources of zinc include meat, chicken, nuts, lentils, and fortified cereals. Zinc is excreted primarily via the gastrointestinal tract with 10 % urinary excretion. When zinc becomes deficient, gastrointestinal and urinary excretion will decline. The obligatory gastrointestinal losses associated with malabsorptive procedures may interfere with the process of reducing gastrointestinal zinc losses in the setting of deficiency [14]. The recommended dietary allowance for zinc is 8 mg/day for females and 11 mg/day for males.
Zinc deficiency is common worldwide and is characterized by impotence, hypogonadism, oligospermia, alopecia, impaired taste, immune dysfunction, impaired wound healing, and various skin lesions. Currently, body zinc status is most commonly assessed by measurement of plasma levels. Plasma levels do not correlate with tissue levels and may not be the best test to assess zinc status. Measurement of red blood cell zinc levels may prove to be superior, but is not commonly performed at present. In plasma, zinc is bound to albumin, and thus any condition such as protein malnutrition or an inflammatory state, which reduces blood levels of transport proteins, will reduce the blood zinc level [14, 15].
Among candidates for bariatric surgery, blood levels of zinc are below threshold in 8–30 % of patients [1, 14, 16, 17]. Among these studies, the lowest incidence of low zinc levels was 8.1 % in a cohort with no abnormalities of albumin or circulating protein [14]. The effects of bariatric surgery on zinc nutrition have not been well studied to date, but the impact of bypassing the duodenum and displacement of the contact of pancreatic enzymes with food and other factors related to bariatric surgery appears to induce a period of negative zinc balance after gastric bypass and malabsorptive procedures. Despite a limited intake of zinc in the first 2 months after gastric bypass, plasma and red blood cell zinc levels are maintained, but urinary excretion declined [18]. A more recent study of 67 women before and then at 6, 12, and 18 months after gastric bypass surgery assessed zinc nutriture with multiple analytical tests and zinc absorption using dual isotopes of zinc at each interval showed that zinc nutritional status slowly deteriorated at each time interval and that zinc absorption was significantly reduced at 6 months with a modest but significant recovery by 18 months (Fig. 8.2) [19].
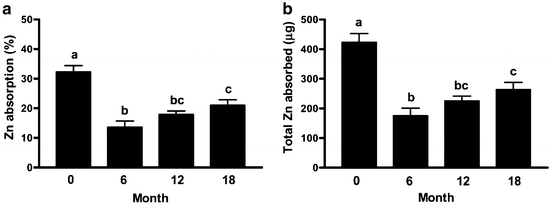

Fig. 8.2
Mean zinc absorption from a standard test meal given before, and 6, 12, and 18 months after Roux-en-Y Gastric Bypass. Reproduced with permission from Ruz M, Carrasco F, Rojas P, cococeo J, Inostroza J, Basi[fer K, et al. Zinc Absorption and Zinc Status are Reduced after Roux-en-Y Gastric Bypass: A Randomized Study using two Supplements. Am J Clin Nut 2011; 94:1004–1011 [19] Copyright © 2014 by the American Society for Nutrition
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








