(1)
Obesity Institute, Geisinger Medical Center, Southold, NY, USA
Abstract
Protein and micronutrient deficiencies are well-known complications of bariatric surgery. Recent evidence suggests that these deficiencies can be present in significant numbers of candidates for bariatric surgery. This chapter will review the importance of protein and vitamin nutrition in relation to bariatric surgery and rapid weight loss. Nutrients are discussed individually in regard to metabolic function, mechanism of absorption, daily requirement, assessment technique, and clinical effects of deficiency. Current recommendations for supplementation and management of deficiency are included.
Although obesity is a manifestation of overnutrition, recent evidence suggests that nutrient abnormalities and deficiency states are not uncommon in individuals suffering from extreme obesity. Clinicians are familiar with starvation-related malnutrition, but may be less familiar with the recognition of deficiencies in protein or micronutrients which are classified as malnutrition associated with chronic disease [1]. Despite the variety and availability of relatively low cost foods in the western world, micronutrient deficiencies are frequent in the severely obese and probably reflect a poor quality diet with high carbohydrate and fat intake.
Many severely obese patients turn to bariatric surgery as the only treatment that offers them a meaningful chance for a longer and healthier life. The association of micronutrient deficiencies with extreme obesity may influence the severity of comorbid disease, the risks of surgery, and the probability of severe deficiency during the period of rapid weight loss and diminished food intake. Bariatric surgery programs will need to expand the nutritional assessment to include an accurate dietary history and more detailed assessment of micronutrient status. Table 7.1 summarizes several current studies of micronutrient status in candidates for bariatric surgery [2–4]. These results suggest that abnormally low levels of many nutrients are unexpectedly common among candidates for bariatric surgery.
Table 7.1
Results of several recent studies addressing nutritional status among candidates for bariatric surgery
Study | Ernst et al. [2] (%), (n = 232) | Ernst et al. [2],(n = 89) | Flancbaum [3] (%), (n = 379) | Schweiger et al. [4] (%), (n = 114) |
|---|---|---|---|---|
Albumin | 12 | 1.1 | 0 | |
Total protein | – | – | 0 | |
Calcium | – | 3.2 | 0.9 | |
Phosphate | 8 | – | 2 | |
Magnesium | 4.7 | – | – | |
Ferritin | 6.9 | 8.4 | 23.9 | |
Hemoglobin | 10.1 | 22 | 18.4 | |
Zinc | 24.6 | – | – | |
Folate | 3.4 | – | 24.3 | |
Vitamin B12 | 18.1 | 0 | 3.6× | |
25(OH) vitamin D3 | 61.2 | 68.1 | – | |
Parathormonea | 36.6 | – | 39 | |
Copper | 0 | – | – | |
Selenium | 32.6 | – | – | |
Vitamin B1 | 0 | 29 | – | |
Vitamin B3 | 5.6 | – | – | |
Vitamin A | 0 | – | – | |
Vitamin E | 2.2 | – | – |
Protein
Strategies for maintenance of adequate protein nutrition for patients in the face of rapid postoperative weight loss and limited food intake remain a challenge for bariatric surgery programs. Lack of attention to protein nutrition in bariatric surgery patients can lead to postoperative complications, interference with comorbidity resolution with weight loss, and impairment of physical function. The human body is made up of two major compartments: body fat (which includes all fat in the body), and fat free mass (which includes bone, water, muscle, and organs). Body protein, a major component of the fat free mass, is constantly turning over with ongoing synthesis of new protein and breakdown of senescent protein. Protein turnover is the balance between protein synthesis and protein breakdown (Fig. 7.1).
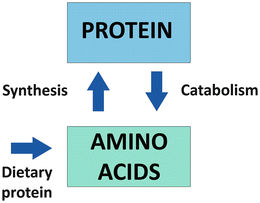

Fig. 7.1
The overall scheme of body protein turnover
Body protein turnover is not a fully efficient process and a daily supply of dietary protein is needed for maintenance of muscle and visceral protein balance. The recommended dietary allowance (RDA) for protein is 0.8 g/kg/day. An anabolic state occurs when protein synthesis exceeds protein catabolism. This can occur in an actively exercising individual whose diet is adequate in protein and energy. When the breakdown of protein exceeds synthesis a catabolic state occurs. The period of rapid weight loss during the first 6–9 months after bariatric surgery is a period of major protein catabolism, which is induced by severe caloric restriction and compounded by limited dietary protein intake. This condition of partial starvation can lead to both loss of fat mass and significant loss of lean tissue with health consequences.
A small percentage of candidates for bariatric surgery may have deficiencies of protein, which can be detected, as low levels of albumin and total protein. Due to the major changes in protein economy following bariatric surgery procedures, a detailed protein nutritional assessment and dietary history as well as diet education focusing on the critical importance of an adequate dietary protein intake needed for healthy weight loss is essential for bariatric surgery candidates. Major preoperative protein deficiencies are best corrected during the preoperative period in order to avoid additional surgical morbidity.
Following bariatric surgery, numerous factors contribute to the risks of poor protein nutrition. All of the procedures limit the gastric acid and pepsin hydrolysis of dietary protein. Gastric bypass and the malabsorptive procedures both bypass the duodenum and proximal jejunum, thus altering the normal environment for digestion and absorption of dietary protein. In addition, anorexia, early satiety, and intolerance to red meat all will limit dietary protein intake. Several studies have demonstrated that dietary protein intake in patients in the first year following restrictive, gastric bypass, and malabsorptive procedures falls well below the RDA for protein [5, 6]. In one study of 101 consecutive patients undergoing gastric bypass or sleeve gastrectomy, protein intake from food and supplements was assessed at 4, 8, and 12 months after surgery. A protein intake of <60 g/day was present in 45, 35, and 37 % of the cohort was found at 4, 8, and 12 months after surgery. Poor compliance with protein supplements was also noted [6]. Major protein catabolism in the first 6–9 months after bariatric surgery is confirmed by body composition studies, which demonstrate 18–30 % losses of lean body mass during this period [7, 8]. Protein catabolism has functional consequences, which include hair loss, fatigue, muscle weakness, and possibly visceral losses.
There is now evidence that a protein intake which is ≥60 g/day or ≥1.1 g/kg/day during the first year after bariatric surgery will result in less protein catabolism and preservation of lean tissue [9]. There is also evidence that a dietary protein intake of ≥1 g/kg/day during the first year after gastric bypass surgery will provide healthier weight loss and preservation of lean body mass [10]. Compliance with a postoperative diet that is adequate in protein is feasible and attainable when appropriate follow-up and dietary education are provided [10, 11].
Additional potential advantages of a high protein diet after bariatric surgery include improved weight loss as a result of an increased energy expenditure, better glucose control, lipid lowering, and reduced weight regain [11]. This is an important area for potential study in bariatric surgery because of the real likelihood that adherence to a high protein diet will enhance many of the established benefits of bariatric surgery.
Candidates for bariatric surgery must be made aware of the critical importance of protein nutrition and protein economy in the health outcome and safety of major weight loss. The capacity to understand this and cooperate with recommendations and follow-up expectations should be a factor in patient selection for surgery. Patients must understand that short-term catabolism is usually without consequence in a well-nourished individual, but longer-term catabolism is dangerous. A postoperative diet containing 60–120 g/day of protein is recommended in nutritional guidelines to maintain fat free mass [11–13]. During close follow-up, if protein intake remains <60 g daily, nutritional supplements are indicated. There is some evidence that dietary enhancement with the branched chain amino acids, especially leucine, may influence the conservation of lean tissue during periods of catabolism. A leucine intake of 10 g per day is recommended after bariatric surgery. Protein sources rich in leucine include whey protein (14 %) and Casein (10.1 %) [11, 14]. Bariatric programs need to engage nutritionists with knowledge of the large array of protein modular supplements to closely monitor patient diet and protein intake following bariatric surgery.
Thiamine (Vitamin B1)
Thiamine deficiency is a well-known and feared nutritional complication of bariatric surgery. Absorption of thiamine takes place in the jejunum and ileum via an active carrier-mediated process. Intestinal thiamine is derived from two sources: diet and generation of the vitamin by the intestinal bacterial flora. The level of thiamine in the extracellular fluid regulates its intestinal absorption. Following absorption, thiamine is phosphorylated and becomes a vital cofactor for steps in glycolysis and the oxidative decarboxylation of carbohydrates. It is also a cofactor for pyruvate dehydrogenase, which regulates the entry of pyruvate into the Krebs cycle. Lack of thiamine is occasionally associated with lactic acidosis because thiamine regulates the conversion of lactate to pyruvate. Because of a short half-life and limited stores, a continuous supply of this vitamin is essential for optimal metabolism. The RDA for thiamine is 1.1–1.2 mg/day [15].
Thiamine nutrition is usually assessed by measurement of levels of thiamine in serum and/or red blood cells. The normal serum level of thiamine is 80–150 μg/dl. A normal level does not exclude the diagnosis of deficiency, and the more definitive test is red cell level of thiamine diphosphate. In the clinical situation, if there is suspicion, empiric treatment is cost-effective and preferable to waiting for results from reference laboratories [15].
Clinical manifestations of thiamine deficiency involve the nervous and cardiovascular systems. Wernicke’s encephalopathy involves ocular abnormalities, ataxic gait, and mental status changes. Congestive heart failure is also caused by thiamine deficiency. Thiamine deficiency is also common in patients taking furosemide in doses of 80 mg per day as furosemide causes increased thiamine excretion in the urine [15]. Thiamine deficiency as determined by low levels has been reported in 15.5–29 % of candidates for bariatric surgery, especially in African Americans and Hispanics [3, 16]. Postoperative deficiency has been reported as early as 4 weeks after bariatric surgery and should be suspected in any patient with poor dietary intake after bariatric surgery. It should also be considered in any patient with cerebral dysfunction after bariatric surgery. Deficiency appears to be less common after restrictive procedures.
Candidates for bariatric surgery should be screened for thiamine deficiency and those with low levels should be supplemented with 100 mg thiamine, two to three times daily. This should be continued for 1 month or until levels normalize [17]. Perioperative supplementation in doses of 100 mg, two to three times daily, should be provided parenterally [3, 17]. Postoperative patients with neurologic symptoms suggesting deficiency should receive aggressive parenteral replacement. Dosage and duration of therapy are controversial, but current recommendations are for 100–200 mg per day for 7–14 days [13, 18]. All bariatric surgery patients should receive a daily multivitamin supplement which contains thiamine.
Ascorbic Acid (Vitamin C)
Vitamin C has an important antioxidant function as a neutralizer of reactive oxygen substances. It also has a major role in connective tissue metabolism, proline hydroxylation, and in facilitating the absorption of heme iron. Dietary sources of vitamin C include citrus fruit and green vegetables. The RDA for vitamin C is 90 mg/day for males and 75 mg/day for females [1]. Vitamin C deficiency causes scurvy with symptoms of generalized weakness, fatigue, and bleeding in skin as well as gums. Vitamin C nutrition is usually assessed by blood levels. Normal values are 0.6–2 mg/dl [1]. Information regarding vitamin C deficiency in bariatric surgery candidates is limited. However, a recent study of 266 consecutive elective general surgery patients included 167 candidates for bariatric surgery (BMI ≥35). In the entire cohort, increasing BMI was associated with a lower ascorbic acid level and 36 % of the cohort was either depleted (level <0.3 mg/dl) or deficient (level 0.3–5.9 mg/dl) [19]. Data about vitamin C deficiency after bariatric surgery is also limited. In a 1-year study comparing patients after gastric bypass and duodenal switch, vitamin C levels increased during the first postoperative year with supplementation. A second study documented falling vitamin C levels during the second postoperative year [20]. Scurvy has been reported in a postoperative bariatric surgery patient with vomiting and poor dietary intake [21].
Bariatric surgery candidates should be screened for vitamin C deficiency. Patients with depletion or deficiency should be treated with repletion doses of 200 mg per day in order to minimize surgical risks related to vitamin C. Postoperative supplementation with a multivitamin preparation provides 60–100 mg. This should be sufficient if postoperative food intake is sufficient. Additional indication for supplementation is to enhance iron absorption. Patients should be made aware that vitamin C supplementation will increase oxalate excretion and the risk of kidney stones [22].
Vitamin B12 (Cobalamin)
Vitamin B12 is a water-soluble vitamin utilized by all cells as a coenzyme for many metabolic reactions. The absorption of this vitamin is complex and involves several areas of the gastrointestinal tract that are involved with bariatric surgery. Cobalamin is ingested bound to dietary proteins. In the gastric lumen, cobalamin is released from its binding to dietary protein and complexes with R-binding protein which is derived from saliva. As part of the gastric response to a meal, parietal cells release Intrinsic factor, which has a binding site for cobalamin that is inactive at low pH. Intrinsic factor accompanies the cobalamin-R-binding protein complex to the duodenum where the cobalamin is released from R-binding protein by pancreatic enzymes. Cobalamin then binds to intrinsic factor at alkaline pH and this complex is bound by enterocytes in the terminal ileum where absorption takes place.
Vitamin B12 is involved in the metabolism of every cell as it participates in synthesis and regulation of DNA. In addition, it has a role in fatty acid synthesis and energy production. In conjunction with folate, it is the cause of the megaloblastic anemia seen with deficiency of either vitamin. Vitamin B12 is also involved in the central nervous system development, myelination, and function. Humans are capable of storing vitamin B12 in the liver with stores between 2 and 5 mg. The daily requirement is 2.4 μg, and the liver stores allow for a long interval between the onset of deficient intake and the development of deficiency symptoms. The initial test to assess the status of vitamin B12 nutrition is measurement of the serum B12 level. Normal values are 200–900 ng/ml. Levels <170 ng/ml even in the absence of symptoms suggest deficiency, and deficiency symptoms are common with levels <100 ng/ml. Both false positives and negatives are common. Confirmation of deficiency may require measurement of serum levels of methylmalonic acid or total homocysteine as these levels are markedly elevated in the setting of B12 deficiency and levels will fall promptly with replacement which allows for monitoring of replacement [23].
In the studies of candidates for bariatric surgery, the prevalence of vitamin B12 levels below threshold is 0–18 % (Table 7.1) [2–4, 24]. Given the complex absorption of this vitamin and the anatomic alterations created in bariatric surgery, it is not surprising that postoperative deficiencies are quite common. The reported incidence of postoperative vitamin B12 deficiency is 26–70 % [12], but most of the studies document only falling serum levels and not proven symptomatic deficiency [24, 25]. Contributors to deficiency include limited postoperative intake of animal proteins, limited gastric acid cleavage of B12 from dietary protein, and diminished production of intrinsic factor [26]. Manifestations of B12 deficiency include macrocytic anemia, leukopenia, glossitis, thrombocytopenia, paresthesia, and irreversible neuropathies [23].
Candidates for bariatric surgery should be screened for vitamin B12 nutrition deficiency. Routine postoperative supplementation with a multivitamin preparation, which contains 6–25 μg B12 per tablet, is not sufficient to prevent falling levels and deficiency. A crystalline B12 supplement providing ≥350 micrograms B12 daily has been shown to increase serum levels in patients with low levels [27]. Treatment of deficiency requires higher doses. In parenteral dosing, about 10 % of the injected dose is retained [23]. Parenteral replacement consists of several 1,000 μg doses during the first week, then weekly until improvement occurs. Subsequent dosing is 1,000 μg monthly [23]. In patients with pernicious anemia, high doses of oral therapy (2,000 μg daily) have been shown to be as effective as parenteral treatment [23




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








