5 Non-ablative laser and light skin rejuvenation
Summary and Key Features
• Non-ablative skin resurfacing is a safe and effective means of improving many aspects of photoaged skin
• While non-ablative skin resurfacing provides modest results, it also provides minimal downtime
• Non-ablative resurfacing alters cellular and non-cellular components of the skin without causing an open wound
• Patient selection requires careful consideration of medical factors as well as patient expectations
• Non-ablative modalities rarely require more than local anesthesia
• When performed with thoughtful consideration, complications are rare
• Photodynamic therapy has been utilized to maximize laser–tissue interaction and subsequent improvement in photoaging
• Treatment of types IV–VI skin requires adjustment of laser parameters to minimize pigment alteration
• Patients can generally return to their normal activities in 1–2 days following treatment with non-ablative modalities
• For patients with limited available downtime, multiple treatments 2–3 months apart may provide an improved result
Introduction
The dermis (and / or deeper epidermis) can be selectively damaged by two basic approaches:
1. Targeting discrete chromophores in the dermis and / or at the dermal epidermal junction, or
2. Using mid-infrared lasers in the range of 1.3–1.55 µm wavelengths, where water absorption is weak enough that relatively deep beam penetration is allowed (there is only 50% beam attenuation at depths of 300–1500 µm).
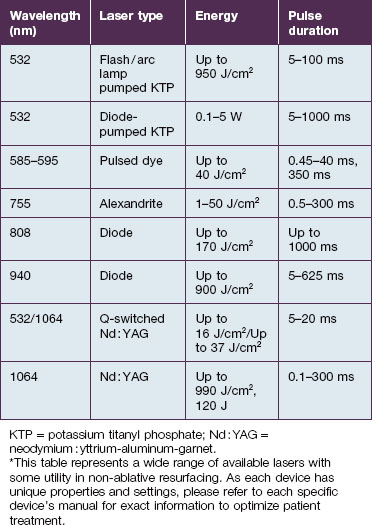
The laser and non-laser systems used for non-ablative rejuvenation are a heterogeneous group of devices that emit wavelengths in the visible (400–760 nm), near-infrared (760–1400 nm), or mid-infrared (1.4–3 µm) ranges, radiofrequency (RF) devices, intense pulsed light (IPL) devices, as well as light-emitting diode (LED) devices (Box 5.1). Each of these modalities can induce dermal remodeling, as well as target other components, without epidermal ablation. Most investigators believe that photothermal heating of the dermis: (1) increases collagen production by fibroblasts and (2) induces dermal matrix remodeling by altering glycosaminoglycans as well as other components of the dermal matrix. Others believe that the laser / light interaction with molecular cellular components alters the cellular function of enzymes as well as cellular structural components. Altering the different components of cells, from enzymes to cellular wall constituents to nucleic acids, may then alter the environment and productivity of a given cell.
Box 5.1
Devices used in non-ablative skin resurfacing
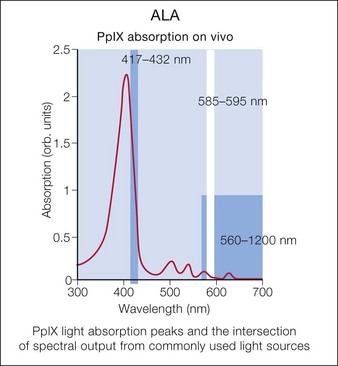
Photodynamic therapy (PDT) with aminolevulinic acid (ALA) has been show to augment the effects of laser or other light sources. Multiple laser and light sources have been used for photoactivation of protoporphyrin IX, leading to improved skin rejuvenation (Fig. 5.1).
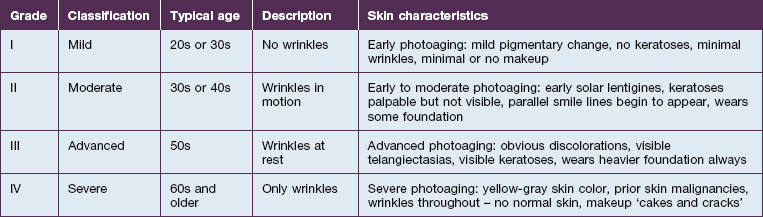
Richard Glogau, MD developed a classification scale to chart the progression of clinical photoaging (Table 5.1). One can follow a patient from an early age, with relatively strong homogeneity of skin coloration and minimal wrinkles, to a more aged patient, with wrinkles at rest and a more heterogeneous skin coloration.
As one would expect, treating a Glogau grade I patient with current non-ablative modalities will achieve a higher percentage of photoaging correction versus more severely photodamaged patients. While ablative skin rejuvenation may achieve superior restoration of normal skin structures, especially for the Glogau grade III or IV patient (see Table 5.1), the downtime and potential risks are prohibitive for many patients. Nevertheless, as non-ablative technologies evolve, restoration of young, healthy skin with diminished risks and negligible recovery times is increasingly possible. The remainder of the chapter will focus on patient selection for non-ablative skin rejuvenation and discussion of the different devices.
Patient selection
Patient selection for non-ablative skin rejuvenation begins with an assessment of the degree and type of photoaging (see Table 5.1). The ideal patient is Glogau grade II or III with mild to moderate photodamage. Non-ablative therapies initiate new collagen formation (collagen I and collagen III) and might be appropriate in a Glogau grade I patient to prevent photodamage progression. Alternatively, a patient and / or a physician expecting dramatic change following a non-ablative rejuvenation procedure in a Glogau grade IV patient may be disappointed.
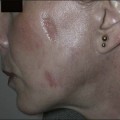
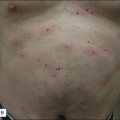
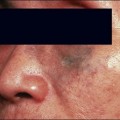
Sadick divides patients in a different manner, where cosmetic deficiencies are based on the histological location of solar damage. His selection process takes into account epidermal (type I) damage (Fig. 5.2) and dermal / subcutaneous (type II) damage (Fig. 5.3), and subsequently treatment is tailored to laser selectivity of the damage.

Figure 5.2 (A–C):Type I photoaging indications.
Republished with permission. Sadick NS 2003 Update on non-ablative light therapy for rejuvenation: a review. Lasers in Surgery and Medicine 32:120-128.
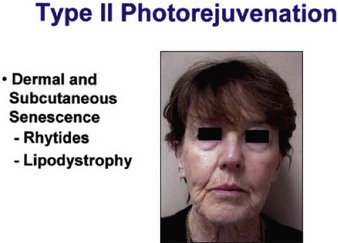
Figure 5.3 Type II Photoaging indications.
Republished with permission. Sadick NS 2003 Update on non-ablative light therapy for rejuvenation: a review. Lasers in Surgery and Medicine 32:120-128.
Another important factor in patient selection is the patient’s Fitzpatrick skin type. Fitzpatrick IV, V, and VI skin types may not be optimal candidates for particular non-ablative rejuvenation modalities that selectively heat melanin. The most common adverse result for non-ablative rejuvenation in darker skin patients is hyperpigmentation, a condition that usually resolves after 4–8 weeks (but can persist longer in some circumstances) with appropriate application of suppressors of melanin synthesis. Mid-infrared lasers, which minimize direct melanin targeting, can be used in patients with darker skin types. However, higher fluences in these patients may result in thermal damage and bulk heating, which can also result in dyspigmentation. Non-cryogen cooling devices can minimize bulk heating, whereas cooling devices that employ cryogen spray may induce pigmentary alterations similar to liquid nitrogen. See Chapter 10 for a detailed discussion of laser and non-laser light sources for the treatment of darker skin types.
Beyond skin type and amount of photodamage, there are some patients who might be excluded from non-ablative lasers and light sources based on medical criteria (Box 5.2). Oral retinoid use, recent rejuvenation procedures, infection, and active dermatitides are reasons to consider deferring a non-ablative rejuvenation procedure. Most likely oral retinoids will not affect the outcome, but no controlled study has investigated their effect on non-ablative skin resurfacing. Many texts advocate waiting a period of 6–12 months, most likely representing an extrapolation from ablative resurfacing wait times. Some cutaneous laser experts have used non-ablative devices 1 month following retinoid use without adverse outcomes.
Box 5.2
Relative contraindications for non-ablative resurfacing
 Active dermatitis (i.e. acne, autoimmune disease, etc.)
Active dermatitis (i.e. acne, autoimmune disease, etc.)
 Active infection (i.e. herpes, impetigo, etc.)
Active infection (i.e. herpes, impetigo, etc.)
 History of keloid / hypertrophic scar formation
History of keloid / hypertrophic scar formation
 History of koebnerizing dermatitis (i.e. psoriasis, vitiligo, etc.)
History of koebnerizing dermatitis (i.e. psoriasis, vitiligo, etc.)
 History of photoinduced dermatitis (i.e. polymorphous light eruption, lupus, etc.)
History of photoinduced dermatitis (i.e. polymorphous light eruption, lupus, etc.)
 History of oral retinoid use in the past 6–24 months
History of oral retinoid use in the past 6–24 months
 Recent ablative resurfacing procedure
Recent ablative resurfacing procedure
 Recent medium or deep chemical peel
Recent medium or deep chemical peel
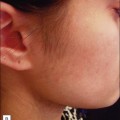
Physicians must also consider the wavelength of the device. For example, devices that utilize visible light (i.e. LED devices, etc.) may exacerbate a phototoxicity / photosensitivity or a systemic condition that is photosensitive, like cutaneous lupus (although in a recent study only 7% of SLE patients reacted to visible light) (Fig. 5.4). On the other hand, some lasers may confer a protective quality. There is increasing evidence that IPL can activate fibroblasts as well as confer protection from future UV-induced skin damage.