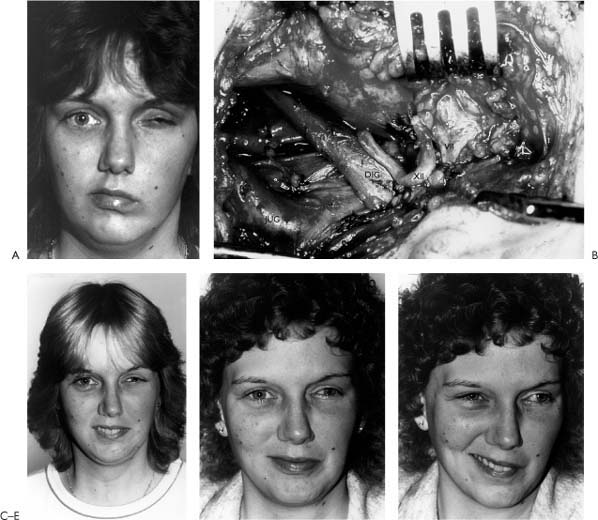
3 There are a number of approaches that qualify as cranial nerve substitution procedures. These include XII-VII crossover, XII-VII jump graft, VII-VII cross-face graft, and XI-VII crossover. Ideally these procedures should be performed when the proximal segment of the facial nerve is not available and within 30 days to 1 year after injury. Although mimetic movement is not possible with these procedures, the majority of patients demonstrate voluntary facial movement with tone and symmetry at rest. Of all the procedures, the “gold standard” is the XII-VII crossover because it gives the most reliable consistent results. For this reason, the XII-VII crossover will be presented with greater detail than the other cranial nerve substitution techniques. However, the general concepts regarding the XII-VII crossover, for the most part, are relevant to the other procedures. Approximately 120 years ago, Drobnik1 (1879) attempted the first cranial nerve substitution procedure, an accessory-facial-end-to-end neurorrhaphy to rehabilitate a patient with an otogenic facial paralysis. In 1895, some 16 years later, Ballance attempted an end-to-side without success. For the next 30 years, there were several isolated reports of attempts to reanimate the paralyzed face using nerve crossover procedures, using predominantly the accessory, glossopharyngeal, and hypoglossal nerves, with only limited success; indeed, the majority of the world was focusing on the accessory nerve as the nerve of choice. In 1932, Ballance and Duel2 summarized the experience with accessory-facial anastomosis stating that the technique invariably gave rise to mass-associated movements of the shoulder and face and that dissociated movements were distinctly unusual and required considerable re-education of motor control. Moreover, they recognized the problems of the functional deficit created by accessory nerve sacrifice. In 1901, Korte3 is perhaps the first to be credited with performing a hypoglossal-facial anastomosis on a woman suffering with a facial paralysis secondary to an infectious petrositis. Two years after the operation, Korte reported a favorable outcome of the surgery. Since Korte’s observation, and certainly for the first third of the 20th century, most surgery for facial paralysis centered around some type of nerve anastomosis, regardless of the site of injury or etiology. During the past 50 years, advances in neuroanatomy, physiology, and surgical technology have permitted more scrutiny in selecting the most appropriate method of reanimation, so that the role of nerve crossover procedures such as hypoglossal-facial anastomosis has become more clearly defined. The idea behind a hypoglossal-facial anastomosis is best summarized metaphorically by the adage, “rob Peter to pay Paul.” Fundamentally, the hypoglossal nerve is sacrificed in order to transfer its motor “fire power” to the facial nerve. The essence of the procedure is to attempt to improve a devastating neurologic deficit at the expense of an iatrogenically created deficit of lesser consequence. The basic question then becomes: how much improvement for what cost? In order to answer this question, one must have an understanding of the clinical and neurophysiologic aspects of hypoglossal-facial anastomosis. This requires a study of the following issues: Hypoglossal-facial anastomosis is most appropriately performed for complete and permanent facial paralysis when the central stump of the facial nerve is unavailable for repair or grafting and when performed up to 2 years after injury. This situation is most commonly seen following cerebellopontine angle tumor surgery in which the nerve is irreversibly injured and not grafted; it also is seen following radical temporal bone, parotid, and skull base surgery for tumors, and following severe temporal bone-brainstem trauma. The requirements for the successful performance of hypoglossal-facial crossover are an intact extracranial facial nerve, an intact mimetic facial muscles, an intact donor hypoglossal nerve, and a patient who can physiologically and psychologically accept the neurologic deficit created by sacrifice of the twelfth nerve. Contraindications to the procedure occur when these criteria are not met. Patients with developmental facial paralysis lack a sufficient population of extracranial facial nerve fibers and facial muscles capable of accepting reanimation, and thus are not candidates for this procedure. Similarly, patients who have undergone massive resections with sacrifice of the extracranial twelfth nerve, including the muscles of expression, would not be candidates. Patients with tumors that notoriously involve nerves such as neurofibromatosis (Type II) or adenoid cystic carcinoma are at risk for bilateral and multiple cranial nerve involvement. These patients would be poor candidates for nerve substitution procedures because of the risk for multiple cranial nerve deficits. Thus, sacrifice of an intact twelfth nerve may compound future functional deficits. A similar situation may occur in patients with a tenth nerve deficits, in whom the loss of one hypoglossal nerve may create an intolerable and potentially crippling problem with swallowing. The significance between time of injury and repair is supported by physiologic, experimental, and clinical data. Physiologic evidence of this concept comes from our understanding of distal nerve degeneration following nerve injury. Nerve biopsy may provide some useful information concerning the potential for success of a nerve crossover procedure, although this is not indicated for most patients. Ylikoski et al.4 studied biopsy specimens of distal facial nerves prior to performing XII-VII anastomosis at time intervals varying from 2 weeks to 2.5 years in five patients. He suggested that success of reanimation correlated with histologic findings in that no recovery of facial function was seen when complete neurofibrosis had occurred in the distal facial nerve prior to anastomosis and varying degrees of functional return were seen in those patients demonstrating Büngner bands (Schwann cell derived tubular structures within a degenerating distal nerve segment that provide guides for regenerating axons). When the time following the injury exceeds 4 years, collagenization and fibrosis of the distal facial nerve is likely too advanced to permit functional axonal regrowth after a nerve crossover procedure. While the state of the facial muscles also plays a role, complete atrophy and myofibrosis likely takes longer to occur than distal neurofibrosis and thus is less of a limiting factor (see Fig. 2-8). Clinical data lend further support to this concept of timing. Gavron and Clemis,5 for instance, found in their review of 36 patients that an interval of more than 1 year significantly affected the outcome of hypoglossal-facial anastomosis. In Conley’s review of 122 patients following hypoglossal-facial anastomosis, the largest single series reported, the quality of recovery also was time dependent.6 Based on the results of their study of 29 acoustic neuroma patients, Kunihiro et al.7 reported hypoglossal-facial nerve anastomosis can be delayed up to 2 years with only minimal effect on the recovery of facial movement. Although it is possible to expect some recovery 2 years after paralysis, recovery after 4 years is unexpected, assuming there was complete separation of the facial nerve and evidence of complete denervation. It is particularly important to differentiate complete, permanent paralysis from partial or reversible denervation. It would be an error to sacrifice a potentially intact or recoverable facial nerve with nerve substitution. The clinician must use all available information such as history, operative records, evoked and spontaneous electromyography, and, most importantly, clinical observations regarding tone and volitional movement in making this determination. This type of dilemma might occur following acoustic tumor surgery where the facial nerve is not transected, but rather is injured by crush, stretch, or cauterization. In such a case where spontaneous recovery is likely, sectioning the facial nerve for nerve substitution should be delayed. Facial nerve regeneration may take as long as 12 to 15 months before any significant sign of recovery is noted. In some cases, the number of facial nerve fibers regenerating through the injured segment is too small to effectively contribute evidence of some recovery. In such cases, the decision of whether to re-explore the proximal facial for grafting, as opposed to performing a nerve substitution, or augmenting existing function with other reanimating techniques that do not impair further functional recovery becomes more problematic. These are types of situations in which nerve substitution performed past the 4 year cutoff might still prove somewhat successful, as the distal facial nerve has not completely neurofibrosed. From a functional and phylogenetic standpoint, it seems plausible that there is a preformed synergism between the facial and hypoglossal nerves, which would therefore justify the selection of this nerve for reanimation.8 Practically, the facial and hypoglossal nerves must cooperate quite closely during speech, mastication, and swallowing. Articulation requires subtle coordination of these two motor nerves for proper motion of the tongue and lips. During food intake, sensory input via the trigeminal nerve must be converted into coordinated motor activity mediated through the facial, trigeminal, vagus, and hypoglossal nerves for effective mastication and deglutition to occur. Although the anatomical proximity of the cortical representation of tongue and facial control is well known, anatomical evidence for interneuronal connections at the central level has only recently been published.9 Electrophysiologic experiments on the trigeminofacial reflex and its correlate following hypoglossal-facial anastomosis, the trigeminohypoglossal reflex, provide further support for the existence of preformed connections via interneurons from the facial afferent trigeminal fibers to both the facial and hypoglossal nuclei. These data regarding linkages between the facial and hypoglossal mononeurons have significant theoretical implications of facial movement following XII-VII crossover.10 Improved facial tone and symmetry occurs in over 90% of patients following XII-VII anastomosis. It is predominantly seen in the midface and to a lesser degree in the lower face, while it is seen least in the frontalis region. Improved facial tone and symmetry is usually seen within 4 to 6 months following neurorrhaphy; however, this is somewhat dependent on time from injury to anastomosis, with better results occurring in earlier repairs.11 Figure 3-1 shows a patient with a complete left facial paralysis following acoustic tumor removal 1 year earlier and postoperatively following XII-VII anastomosis. Voluntary facial movements begin sometime after tone develops and progressively improve over 18 months, although continued improvement has been observed up to 5 years following repair. Voluntary motion is best noted in the midface, and less so in the lower and upper face. The precise reason for this phenomenon has not been demonstrated conclusively, but probably relates to more potential axon pathways destined for the peripheral facial muscles in the midface and less in the forehead, lower lip, and neck. Clipping some of the “excess” midfacial fibers in an effort to redirect regenerating axons to the periphery has been suggested by Fisch12; however, in the author’s experience this has not been substantiated. True spontaneous reflexive facial function or emotional expression is rare, although with training many patients will develop spontaneous animation with speech. Indeed, motor sensory re-education and practice will assist the patient to more effectively learn to use the new system; this is accomplished by teaching the patient to push the tongue against the incisor teeth or palate when attempting to voluntarily smile. Further, they are taught to suppress the normal side while balancing it with the less controlled and weaker abnormal side to improve symmetry. With practice, concentration, and motivation, roughly 10% of patients evolve a behavioral response that habituates and simulates normal smiling13. Although uncommon, true segmental separate regional facial movement control (i.e., eye from mouth) may occur. Conley suggested that this was seen more often following immediate repair.6,10,11,13 Extensive training and motivation can improve the patient’s chances of achieving such an excellent result. Unfortunately, as with any nerve grafting procedure including XII-VII, synkinesis of some degree occurs in virtually all patients. Moreover, mass movement complicates voluntary expression in upward of 80% of patients. Excessive facial tone, hyperactivity, and/or spasm may occur in up to 15% of patients, but this is rarely sufficiently severe to warrant a patient’s request to have the anastomosis undone.6 While not all agree, some suggest that this phenomenon occurs more following early or immediate nerve crossover, thus allowing the overly powerful twelfth nerve axons to maximally reinnervate the face.6 Qualitative and quantitative assessment of overall improvement in facial function is necessary with any technique of facial reanimation. Table 3-1 provides a grading system suitable for evaluation of nerve substitution procedures. Clearly, some degree of synkinesis will be seen with even the best result. A review of the literature shows that not all authors utilized a common grading system; thus an accurate comparison of results is not entirely possible. Conley reported good or excellent results in 65% of 122 patients, fair in 18%, and poor in 17%. He noted that good results increased when nerve substitution was performed immediately (74%) as opposed to delayed (41%). These data were again updated and corroborated by Baker who reviewed Conley’s and his experience with more than 200 patients.13 In 54 patients undergoing 12-7 anastomosis, Luxford and Brackmann reported excellent results in 22%, good in 31%, fair in 28%, and poor in 7%. Six were not available for follow-up.14 In their series, Pensak et al.15 reported good to excellent results in 42%, fair in 48%, and poor in 10%. Further, they demonstrated that most patients (42%) showed signs of reinnervation within 6 months and that 76% manifested signs of reanimation with 1 year. Virtually all of their patients underwent nerve substitution immediately or shortly after nerve sacrifice. Gavron and Clemis5 reported good to excellent results in 61%, fair in 17%, and poor in 6% of 36 patients who underwent hypoglossal-facial anastomosis. They noted that fair to poor results occurred more often in patients over the age of 60 and in patients whose interval to repair was over 1 year. Our own experience with 11 patients agrees with the results reported with these larger series. Sectioning of the hypoglossal nerve unequivocally leads to hemitongue paralysis. However, according to Conley, the degree of hemitongue atrophy varies: 22% minimal, 53% moderate, and 25% severe. Functional disability is also variable. Conley noted that patients undergoing immediate anastomosis rarely complained of dysfunction, whereas those with long-standing paralysis with flaccidity experienced some disability from food lodging in the buccal sulcus or difficulty moving the bolus posteriorly. This usually improved as facial function improved. Postoperative difficulty in swallowing that was attributed to tongue dysfunction was a complaint of 10 to 12% of patients. Articulation is affected in all patients initially, but is rarely disabling and usually improves over time. In studying perceived oral dysfunction following XII-VII anastomosis, Pensak et al.15 noted that 74% of patients reported some difficulty with eating, while only 21% found it debilitating. Rarely was swallowing significantly disturbed. Figure 3-1. Results of hypoglossal-facial anastomosis. Repair was performed 1 year after acoustic tumor resection. A, There was no facial function on the paralyzed left side and vision was limited by the medial and lateral tarsorrhaphies. B, Hypoglossal-facial nerve anastomosis was performed. DIG, digastric muscle; JUG, jugular vein; XII, hypoglossal nerve; VII, facial nerve. C, Six months following the hypoglossal-facial crossover, eye closure improved so that the medial tarsorrhaphy was lysed. Patient regained facial symmetry in repose. D, With the tongue protruding against the teeth, the patient is able to produce a voluntary smile. E, Mimetic or spontaneous smile. Note there is no movement on the involved side. Mimetic facial expression is rarely restored with a nerve substitution procedure. In an effort to reduce the likelihood of postoperative tongue atrophy and dysfunction, Rubin suggested interdigitating the midline tongue musculature using a Z-plasty technique.16 Presumably, this increases the likelihood of muscle-to-muscle neurotization. Conley tried suturing the descendens hypoglossi branch to the distal stump of the hypoglossal nerve in two patients. No recovery occurred in these patients.11 The number of axons available with the descendens hypoglossi is far to few to expect any meaningful recovery (Fig. 3-2). The possibility of splitting the hypoglossal nerve by a microseparation and retrodissection technique and performing a split XII-VII anastomosis was also suggested by Conley who performed the procedure in 12 patients. None of the patients achieved useful facial recovery, and all had tongue deficits.11 Splitting the hypoglossal nerve arbitrarily across a funiculus where fascicles cannot be separated dener-vates the part that is to serve as the donor as well as part of the nerve that is to be spared (Fig. 3-3). The result is decreased viable fibers available to reinnervate the face as well as the tongue. Ueda et al.17 described a technique using a part of the hypoglossal nerve to motor a free muscle transplant. The hypoglossal nerve in its terminal course divides into three branches. The central branch is large, and Ueda and coworkers reported splitting this branch by microdissection and using half of it to motor the free muscle transplant. They were able to show useful reinnervation with serviceable contraction of the free muscle motored by this branch, but tongue atrophy was more or less seen in all the patients. The authors noted that patients had changes in speech and difficulty with handling food and swallowing. In two of their patients, the functional disturbance was severe. The authors commented that functional disturbance caused by resection of the hypoglossal nerve is unpredictable. In two of the patients, they tried the longitudinal division approach described by Kesseler et al.18 The functional recovery in those cases was so poor that they have abandoned the technique. We have observed this procedure performed by a colleague and studied three of these patients immediately and up to 2 years postoperatively. Two patients had tongue paresis, and one had hemiatrophy. The recovery was only fair in these patients in spite of surgery performed within 3 months of injury. The concept of splitting the hypoglossal to spare the tongue is flawed, and the use of the procedure is discouraged.
Nerve Substitution Techniques: XII-VII Hook-Up, XII-VII Jump Graft, and Cross-Face Graft
Mark May, M.D.
Hypoglossal Facial Anastomosis
History
Hypoglossal-Facial Anastomosis (XII-VII Crossover)
General Concepts
Indications, Timing, and Considerations
Anatomical and Physiologic Aspects
Expectations and Results of Hypoglossal-Facial Anastomosis
Disturbance of Tongue Function
| Grade | Definition |
| I – Superb | Some mimetic (spontaneous emotion) movement. Individual movement. Complete eye closure and asymmetrical smile with maximal effort. |
| II – Excellent | Mimetic movement absent. Otherwise same as Grade I. |
| III – Good | Mass movement. Eye closure complete and asymmetrical smile with maximal effort. |
| IV – Fair | Incomplete eye closure and/or very weak mouth movement. |
| V – Poor | Symmetry only. Tone intact. |
| VI – Failure | Flaccid. Tone lost. |
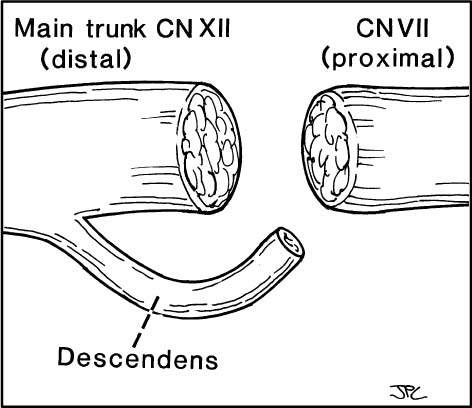
Figure 3-2. Descendens hypoglossi-facial anastomosis. The cut ends of the twelfth nerve, descendens hypoglossi, and distal end of the facial nerve at its extracranial main trunk are depicted approximately to scale. Note that when the facial nerve is sectioned within 30 days following injury, the cross-sectional endoneurial surface of the facial nerve in this circumstance makes a very close match with the end of the hypoglossal nerve. The surface area offered by the descendens hypoglossi is significantly smaller. If the descendens instead of the hypoglossal nerve was used to innervate the facial nerve, there would be a mismatch. The endoneurial surface of the descendens provides inadequate number of axons or “fire power” to expect any useful facial recovery from this procedure (see Chapter 2).
Surgical Anatomy-Hypoglossal Nerve (Fig. 3-4)
The hypoglossal-facial crossover technique requires the identification of the facial as well as the hypoglossal nerve. The course of the hypoglossal nerve can be divided into vertical descending, horizontal, and ascending segments. The anatomical relations of the vertical descending portion of the hypoglossal nerve are relatively constant. Holling-shead18 notes that the twelfth cranial nerve emerges from the hypoglossal canal medial to the internal jugular vein and the carotid artery. It runs laterally and inferiorly to lie behind the tenth cranial nerve, then courses more inferiorly to pass between the internal jugular vein and the internal carotid artery (Fig. 3-4A). In 8% of individuals, the twelfth cranial nerve passes behind the internal jugular vein.19 Another variation is that the nerve may be fused by fibrous adhesions to the nodose ganglion of the tenth cranial nerve in its vertical segment. The twelfth cranial nerve then travels deep to the posterior belly of the digastric muscle and over the internal carotid artery (Fig. 3-4B).
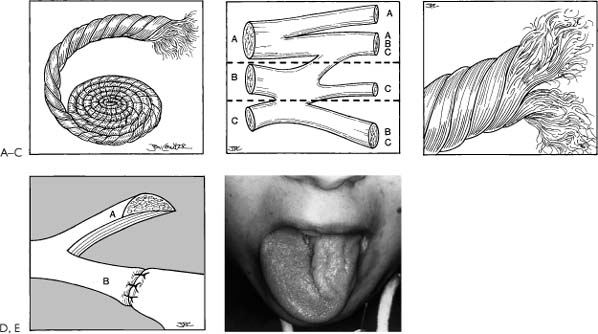
Figure 3-3. Hypoglossal nerve split for facial reanimation. Splitting the hypoglossal nerve in an effort to spare the tongue and reinnervate the face is a flawed concept. The hypoglossal nerve, like all peripheral nerves, is not oriented in parallel channels but rather is random and interwoven much like a rope (A). B, A wax reconstruction of serial sections of a peripheral nerve shows the random mixing of fibers.39 A split between A and B would leave only A fibers; a split between B and C would leave only C fibers. C, Demonstrates the effects of splitting a rope where the fascicles are in a random pattern; therefore, splitting injures the upper and lower part of the nerve separated by the split. In this fashion, the viable axons that would course to the tongue are diminished as well as those to the face. This pattern is more characteristic of the anatomy of the twelfth nerve as opposed to D where it suggests that all the fibers are parallel. In such a case, it would be conceivable that splitting the tongue would achieve the surgical goal of getting maximum innervation to the face without paralyzing the tongue. Unfortunately the rope concept is valid. Thus, a hypoglossal split is a flawed concept and should not be performed. E, Shows a paralyzed and atrophic left hemitongue as a result of splitting the nerve. This is the same effect as one frequently observes with a formal XII-VII where the twelfth nerve is sectioned completely.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








