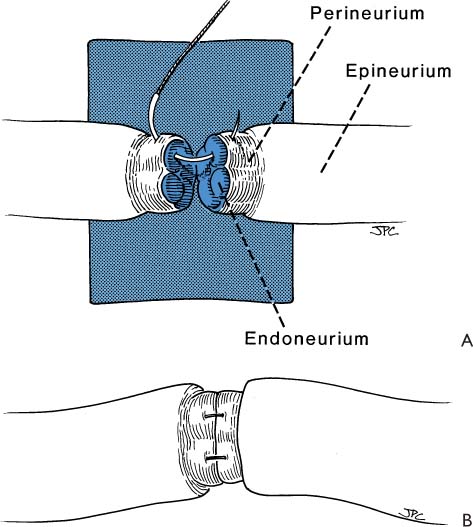
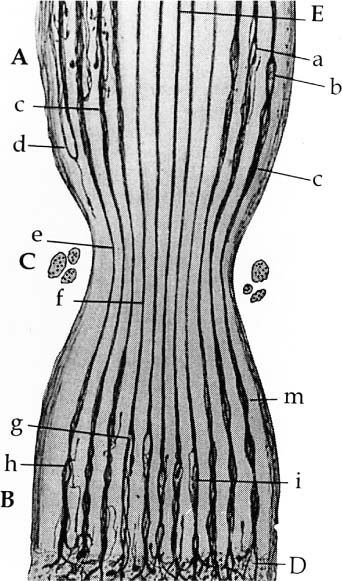
2 Sdantiago Ramon Y Cajal (Fig. 2-1) (born in Petilla De Aragon May 1, 1852; died in Madrid October 17, 1934) was known principally for his histologic documentation of the contiguity of the neurons originating in the central nervous system, which was contrary to the common belief at that time (Fig. 2-2). His scientific achievements earned Cajal numerous honorary awards, including the Medal of Helmholtz in 1905 and the Nobel Prize in Stockholm in 1906. Cajal, with his iron will, continued his scientific pursuit for truth in spite of resistance from his colleagues who initially refused to accept a “new” concept; even the church threatened excommunication if he continued his practice of cadaver dissection. Cajal, an eminent Spanish biologist, philosopher, artist, writer, and clear and insightful observer, was generous with his time, serving as a guide to his students, and was known above all for his simplicity and modesty. Figure 2-1. Santiago Ramon Y Cajal (1852–1934). Nobel Laureate-1906. Pioneer in the neurohistopathologic mechanism of regeneration. Figure 2-2. Histologic preparation of degeneration and regeneration of peripheral rabbit nerve following ligation. The ligature (C) has been in place 8 days. Note central fibers (E) are undamaged. Multiple axon sprouts form at regenerating distal end (D). (Scientific work by Cajal.) Direct repair of the facial nerve is the most effective method of rehabilitating the paralyzed face. Re-establishing a connection between the facial nerve nucleus in the pons and the facial muscle motor unit end plate by nerve repair or an interposition graft is the only technique that will reestablish spontaneous involuntary (mimetic) facial expression. General principles regarding indications, the importance of timing, and expected results are discussed in Chapter 1. Nerve repair or nerve grafting has the advantage of reestablishing direct facial nerve continuity, with all of its obvious anatomical and physiologic advantages. This repair is best done at the time of the primary ablative operation when this is realistic or ideally within 30 days. Otherwise, the anatomy, technical facilities, and wound physiology will never present themselves as favorably. Delayed grafting incorporates all the disadvantages of re-entry through a distorted scar tissue bed where identification, dissection, and delicate repair can prove to be exceptionally difficult. The basis for the surgical approach to nerve repair that is to follow is based on established basic fundamentals covered in detail in Section I of this book. Nerve repair has evolved through rather gross attempts at approximation to the present technique, which incorporates the use of a microscope, specific instrumentation, and 8–0/10–0 monofilament suture material (Table 2-1). Once the ends of the injured facial nerve are identified, the ends are trimmed, the epineurium is peeled back, exposing the endoneurial surface. At this point, the ends of the exposed nerves are stained with 1% methylene blue. This is accomplished with the ends of a wooden cotton applicator dipped in the dye and then touched to the ends of the exposed nerves. This maneuver is quite helpful because the connective tissue component of the epineurial sheath stains dark blue, while the endoneurial surface stains a lighter blue. I have found that using a small amount of dye placed carefully on the nerve endings without allowing the stain to extend to surrounding tissues helps to distinguish the nerve endings from otherwise “look alike” structures such as vessels and connective tissue. Once the ends of the nerves to be repaired are isolated and stained, they are freed up for several millimeters so that a blue background paper can be placed under the nerve thus creating a work platform that stabilizes the nerve and facilitates retrieval of the delicate needle used for nerve repair (Fig. 2-3A). Usually one or two perineurial sutures are all that is required between the main trunk or peripheral branches of the nerve and the donor graft (Fig. 2-3A). One should not lose sight of the fact that the facial nerve has a great capacity for regeneration, and if the nerve endings are approximated and matched without tension in a normal tissue bed, great numbers of axons will sprout and reinnervate the paralyzed muscles (Fig. 2-3B). A number of suggestions have been proposed to increase recovery of facial function and discourage the development of synkinesis: (1) clipping nonessential branches of the facial nerve,1 (2) rotating the donor nerve graft so that the distal end is sutured to the proximal end of the facial nerve, (3) orienting the interposition grafts to match the appropriate segment of the facial nerve,2 and (4) suturing fascicles to fascicles3 (Fig. 2-4). It is of interest to note that the efficacy of all four of these has been challenged, and it most likely makes no difference whether the peripheral branches are clipped, the sural nerve graft is rotated, the cable grafts oriented,4 or the nerve repaired by the fascicular technique.5,6 Further, in the author’s experience, the results achieved were the same whether or not these procedures were followed (see Chapter1).
Nerve Repair
Mark May, M.D.

Introduction
|
* Padgett Instruments, 2838 Warwich T.F. WY, Kansas City MO 64108
†Xomed, 6743 Southpoint Drive, Jacksonville, FL 32216
Perhaps there is a genetic hypothesis that can explain these observations. I would propose that the same influencing factors present in the embryo that bring about the connection between the facial nerve system to the appropriate muscle group is operative to some extent following the completion of embryogenesis. Therefore, the regenerating peripheral facial nerve fibers seem to be genetically programmed to more or less reach their appropriate muscle territory regardless of the orientation at the repair site. Further, the postregeneration synkinesis-hyperkinesis following facial nerve injury may be related more to regeneration morphologic events that occur in the facial nucleus than what occurs in the peripheral axons7–9.
Figure 2-3. A, The ends of the nerves to be repaired are placed over a work platform created by blue background paper. The ends are stained with methylene blue (MB). This set-up provides a dry field and makes it easier to identify and to manipulate the ends of the nerves for surgical repair. To suture donor to recipient, the epineurium is peeled back to expose the protruding endoneurial surface. An 8-0 monofilament suture is placed through the perineurium and out the endoneurium of the recipient and then through the endoneurium and out the perineurium of the donor. Three knots are tied in order to prevent the suture from unraveling. Usually one or two sutures are necessary, depending upon the size of the ends of the nerve repaired and whether there tends to be rotation or a need for improving nerve end approximation. B, The ends of the donor and recipient nerves should be brought together without tension, and the endoneurial surface of these should match so that a maximum number of axons have an opportunity to pass through the nerve graft.
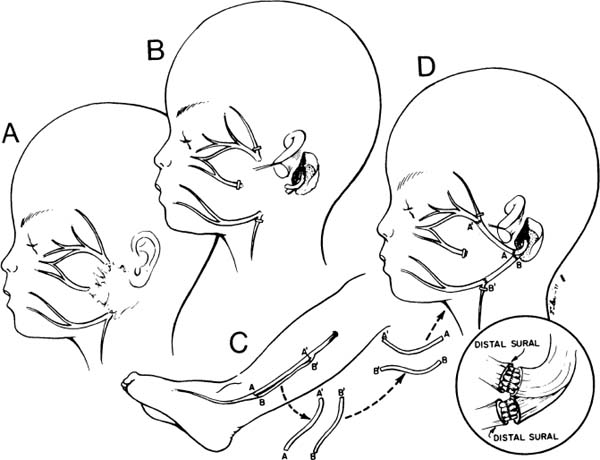
Figure 2-4. Nerve repair strategies intended to improve outcome. A, The lesion is resected. B, Branches to the forehead and extra branches to midface and neck clipped to discourage faulty regeneration and growth of axons to unessential areas. C, Sural cutaneous nerve graft. D, Nerve reversed so that the distal end of the graft is attached to the proximal end of the donor nerve. Insert demonstrates fascicular grafting: orienting the fibers parallel to the topographical orientation of the fibers as they leave the temporal bone. In our experience, none of these techniques illustrated in this figure or the insert have improved the outcome of surgery, even though they have been recommended by other surgeons. (From May M.36 With permission.)
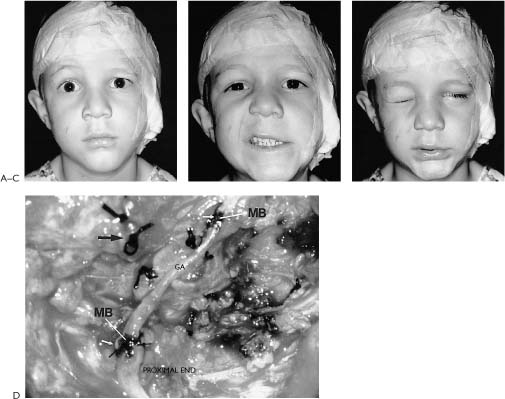
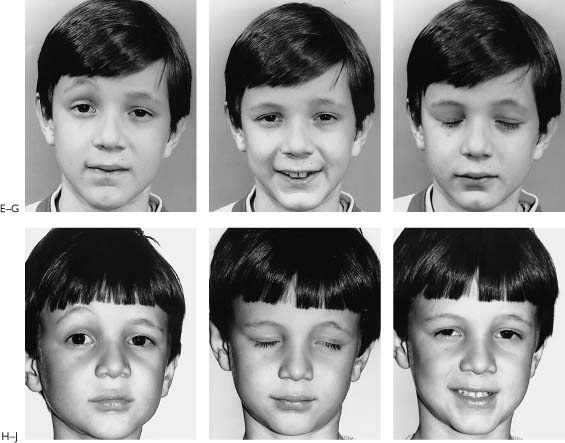
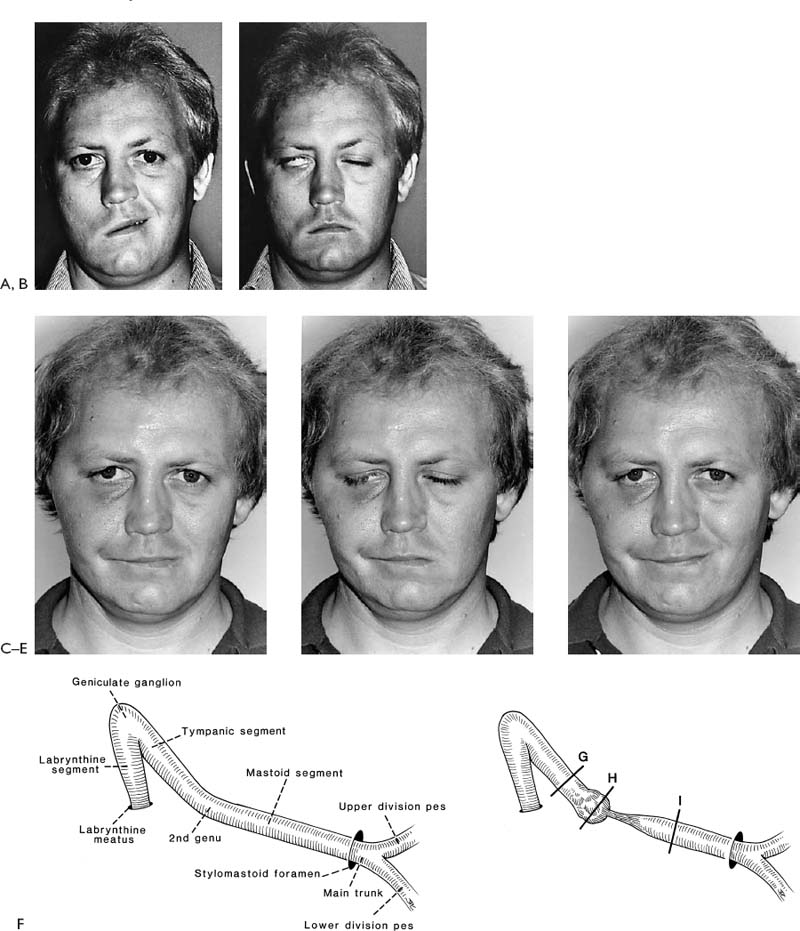
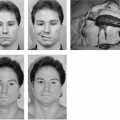
Figure 2-5. This youngster presented 24 hours following an accidental fall through a plate glass window. He sustained a facial laceration that divided the upper division of the facial nerve on the left side. There was paralysis involving the left forehead, brow, eyelids, midface, and upper lip with sparing of the corner of the mouth, lower lip, and neck. A, Twenty-four hours after facial nerve repair, raising the eyebrow. B, Smiling. C, Closing the eyelids tightly. D, At the time of surgery, a gap between the upper division of the facial nerve just beyond the pes was repaired with a 4-cm graft taken from the great auricular nerve. A nerve stimulator is useful in finding the ends of the upper division in its distal segment. The ability to electrically stimulate the distal end of the severed nerve up to 3 days after injury is one of the major incentives for early exploration. Further, upon opening the wound and washing away the clot, the anatomical structures are easier to find as opposed to waiting 10 days or more when the stimulator is not useful and scar tissue is beginning to envelop the site of injury. Note: (1) The great auricular nerve (GA) makes a good match to reconnect the two ends of the upper division of the facial nerve. (2) Methylene blue stains the anastomotic sites (MB). This helps identify the endoneurial surfaces. (3) Compare the 8–0 neurorrhaphy sutures (small arrow) to the 4–0 silk sutures used to tie bleeding vessels (large arrow). (For results of nerve repair see continuation of figure on next page.) E, One year after surgery, raising the eyebrow. F, Smiling. Note that there is some synkinesis as the involved upper brow elevates with an effort to smile. G, Closing the eyes. H, Two years after surgery. There is still inability to raise the brow. This is not unusual following nerve repairs. Upper division repairs frequently do not restore eyebrow function and lower division repairs often leave a lower lip and platysma deficit. I, Closing the eyes. Note sykinetic elevation of the brow on the involved left side. J, A strong symmetrical smile. (From May M et al.17 With permission.)
Factors That Influence Results
The major factors influencing results involve: (1) the time postonset until repair, (2) the condition of the nerve at the time of repair, (3) the presence of tumor involving the facial nerve and/or reactive scar tissue, and (4) adherence to surgical principles of nerve repair.
Timing
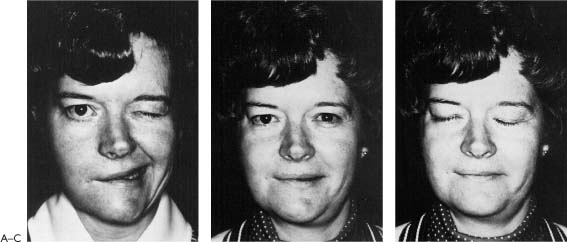
As has been mentioned and stressed in Chapter 1, of all of the factors leading to the best results, timing of the repair is the most important. When the nerve is repaired at the time of injury to within 30 days, the results have been uniformly excellent, yielding mimetic movement, the ability to close the eye with minimal mouth movement, the ability to smile with minimal eye movement, and normal tone and symmetry at rest (Fig. 2-5). The proximal and distal portion of the interrupted nerve between time of onset and 30 days is best suited for repair in terms of its neurobiology as described in Chapters 4 and 8 (Fig. 2-6). Modern neurobiologic techniques have demonstrated that the regeneration process begins within hours from the time of injury.9,10 Axon sprouts are being pushed out from the central stump within 4 days11 and will seek the distal motor unit end plate re-establishing connection between the pons and the facial muscles at a rate of 1 to 3 mm/day. The window of opportunity can be extended to 6 months with a fall-off in the strength of muscle movement recovery due to less axons reaching the motor unit end plates. There is evidence to suggest that the progressive decrease in facial function recovery is related to alterations in the biology of the facial nerve nucleus in the pons as well as shrinking and collagenization of the peripheral system of axons that are distal to the injury (Fig. 2-7). Fisch and Rouleau12 demonstrated evidence of recovery in patients grafted 18 to 36 months after injury but clearly achieved the best results when the nerve was repaired within a year. Our overall results of facial nerve repairs after 1 year have been so disappointing that other techniques such as the hypoglossal crossover or muscle transposition are preferred (Fig. 2-8).
Condition of the Nerve
In addition to the time factor, the cause of the injury must be considered in predicting results. Upon histopathologic examination, a malignant tumor may actually show evidence of neural invasion even with little or no clinical evidence of facial weakness (Fig. 2-9).
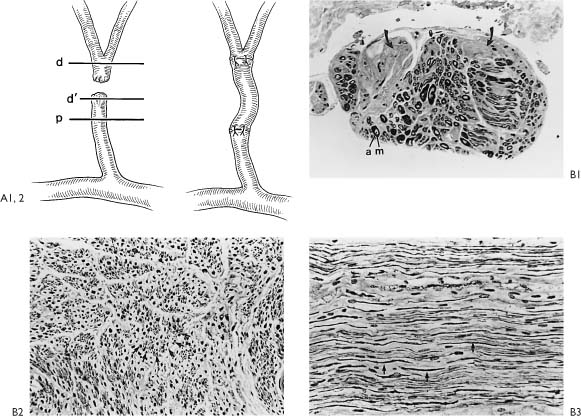
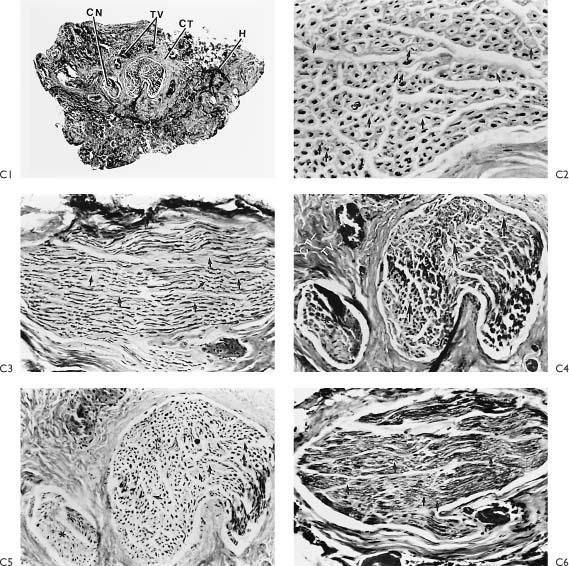
Figure 2-6. A1, A schematic of Figure 2-5D indicates that the injured portion of the proximal and distal end were trimmed back to normal-appearing nerve confirmed by frozen section control (p, proximal; d’, section from end of proximal segment; d, distal segment). A2 demonstrates that a 4-cm graft taken from the great auricular nerve was required to fit between the proximal and distal end without any tension. Note how the small gap becomes significantly larger after the injured proximal and distal segments are trimmed. Prior to placing the graft, frozen section control confirms that following trimming the proximal end shows normal axons and the distal end is beyond the acute injury. B1–3, Are nerve biopsies taken through the proximal end of the injured upper division. This section is proximal to the injury. B1, Cross-section, proximal end (Toluidine blue, ✖42). The specimen is completely normal except for the two sections (arrows) with crush artifact or extension of the injury to involve two fascicles of this multifasciculated nerve bundle. Toluidine blue selectively stains myelin. m, myelin; a, axons. B2, Cross-section, proximal end (Bodian stain, ×250). Bodian selectively stains axons. Both B2 and B3 show a normal axon pattern on cross- and longitudinal section. The arrows point to the axons. B3, Wallerian degeneration involves the proximal end a very short distance, whereas there is complete degeneration distal to the injury. It is important to trim back the traumatized nerve proximally until normal nerve is demonstrated, as depicted in B3. Within 3 days, these axon processes will be extending out the distal end and entering the graft. These processes will be drawn into the graft toward the motor end plate by neurotrophism and guided by the Schwann cell-lined tubes created as the axon and myelin degenerates within the nerve graft and the peripheral neural system to which the graft is attached. C1, Cross-section, distal end through injury (Toluidine blue, ×42). This section is through the acute injury just distal to the traumatic transection as depicted in A1. Note the hemorrhage (H), increased connective tissue reaction (CT), granulation tissue (G), thrombotic vessels (TV), and crushed nerve (CN). Upon review of this specimen, one can appreciate the importance of removing the area of nerve close to the injury and suturing the graft to a healthier appearing nerve more distally. C2–6, These specimens were taken distal to the lesion as depicted in A1. C2, Stained selectively for axons and shows early Wallerian degeneration has already begun within 24 hours of injury. There is some loss of axons (arrows). C3, Clumping and beading of axons indicates early Wallerian degeneration. C4, Trichrome stain for myelin shows vacuolization (arrows), while the general morphology and organization is intact. C5, Loss of axons (arrows). C6, Vacuolization of myelin depicted with the Toluidine blue stain. These histologic specimens demonstrate vividly the favorable situation for nerve regeneration and an anatomicophysiologic basis for early nerve repair.
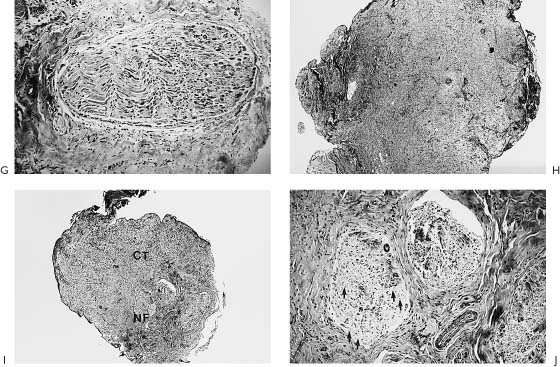
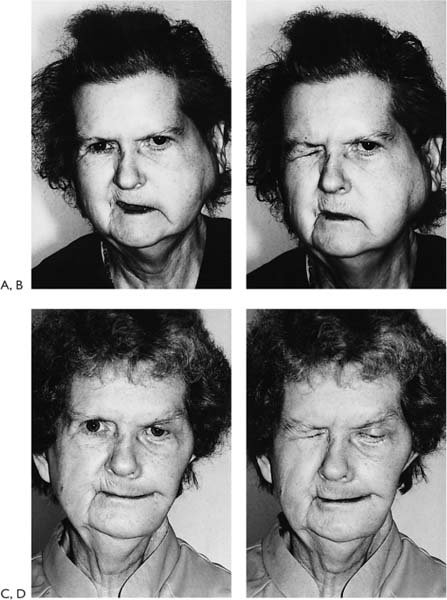
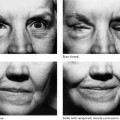
Figure 2-7. Patient with right-sided facial paralysis 5 months following self-inflicted gunshot wound to area of right mastoid tip. A, Smiling, B, Closing eyes. C, D, One year post-nerve graft, temporalis muscle transposition, and eye spring insertion. C, In repose. Note return of tone and symmetry. This is due to the effect of the temporalis muscle as well as nerve regeneration through the nerve graft placed 1 year prior. D, Closing eyes. Note with effort to close the eyes, there is some synkinetic movement of the corner of the mouth on the involved right side indicating nerve regeneration. E, Smiling as a result of voluntary muscle contraction through nerve regeneration as well as contraction of the temporalis muscle. F(left), Schematic representation of the facial nerve within the right temporal bone. F(right), Indicates the levels where nerve specimens were obtained for histologic study. G, Cross-section through tympanic segment proximal to level of injury. Mason trichrome stain, ×33. The stain specific for myelin shows surviving normal-appearing myelin proximal to the injury. The proximal end of the facial nerve will retain its morphology and remain viable for the life of the individual. This intact proximal nerve has the potential to reinnervate the distal system. Unfortunately, the distal facial nerve system collagenizes and is replaced by scar tissue. H, I, J, This process begins as soon as Wallerian degeneration has been completed within 10 to 21 days and progresses rapidly over 3 months. Although the distal system may not be serviceable for reanimation, certainly the proximal surviving end of the nerve could be used to motor a free muscle graft. This approach has been used effectively in two patients. H, Cross-section. Mason trichrome stain, ×8. Section through the traumatic neuroma. There are no identifiable fascicles but rather a haphazard pattern of interdigitating fibrous tissue of a reactive or traumatic neuroma. I, Cross-section. Mason trichrome stain, ×8. Specimen was obtained distal to the traumatic neuroma and shows three fascicles with marked increase in connective tissue encompassing these nerve bundles. CT, connective tissue; NF, neural fascicle. Note the amount of neural shrinkage and increase in connective tissue just 5 months following the injury. J, Cross-section. Mason trichrome stain, ×33. High-power view of the area depicted in I. There are no surviving axons or myelin. The organization and distribution of the neural elements seem to be constricted by surrounding connective tissue. There is already significant collagenization. These factors interfere with regeneration following nerve repair. Considering the amount of shrinkage and collagenization involving the nerve distal to the injury at 5 months, we do not rely on the nerve graft alone and use combination procedures in such a case. In this particular patient, eye reanimation was critical in order for him to return to work as a truck driver. Based on this, an eye spring was inserted to improve eye closure, and a temporalis muscle transposition for mouth function was performed along with a nerve graft. The amount of recovery achieved with the nerve graft was minimal yet significant in terms of tone and some mimetic movement. The combination of procedures complimented each other.
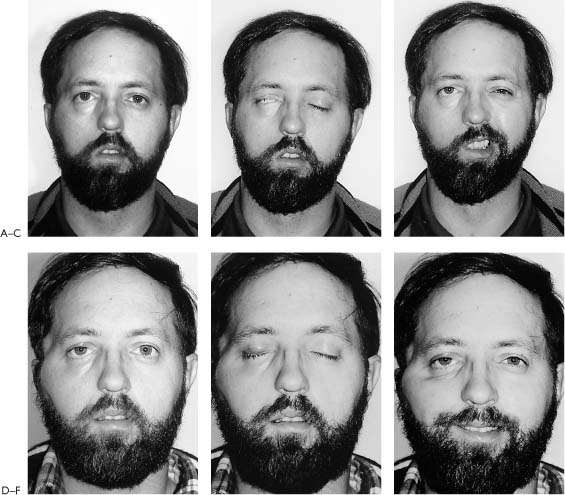
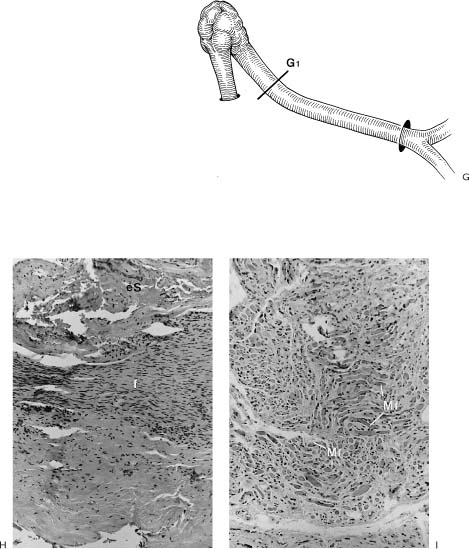
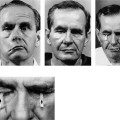
Figure 2-8. Patient presents with an 18-year history of facial paralysis that began slowly over a period of 5 years and then became complete. A meningioma involving the facial nerve at the geniculate ganglion was confirmed at the time of surgery. A temporalis muscle transposition and gold weight implant was used to reanimate the face rather than depend on a nerve graft in a patient who has been paralyzed for 18 years. A, Note widened interpalpebral fissure and some sagging of the corner of the involved right side with flattening of the lip cheek crease. B, Inability to approximate the eyelids on the involved right side. C, No movement of the involved side with smiling. D, Six weeks post-temporalis muscle and gold weight implant. Face in repose. E, Closing the eye. F, Smiling. G, Schematic of the facial nerve within the temporal bone showing the location of the tumor and the site of the specimen (G1). H, A biopsy of the facial nerve was taken distal to the tumor. It shows thickening of the epineurial sheath (eS) with complete collagenization of the endoneural compartment (f). No viable nerve elements were identified by special staining techniques. I, Biopsy of facial muscles in the area of the lip cheek crease shows viable muscle fibers (Mf). The fact that facial muscles survive over long periods of time does not correlate in any way with return of function following nerve repair. A possible explanation for survival of muscle fibers in spite of complete facial nerve denervation may be reinnervation from nerves in the vicinity. It is possible that fifth nerve fibers reanimate the denervated facial muscles. This has been demonstrated by Silverstein et al.35 in a patient with a teratoma involving the facial nerve in the temporal bone. A teratomatous tumor was removed with the facial nerve. Following this procedure, the patient demonstrated spontaneous recovery. It was established that the recovery was through fifth nerve fibers because the movement could be interrupted by blocking the pathway of the fifth nerve by injecting Xylocaine into the region of the foramen ovale. This finding of muscle survival in the face of long-term facial nerve denervation points out the lack of clinical application of muscle biopsy to determine whether nerve repair is a reasonable option.
Figure 2-9. A, Patient with a left-sided facial weakness present 2 months due to occult adenoid cystic carcinoma of the parotid gland. B, High-power view of the specimens from this patient demonstrating both intra- and perineural invasion of the nerve.
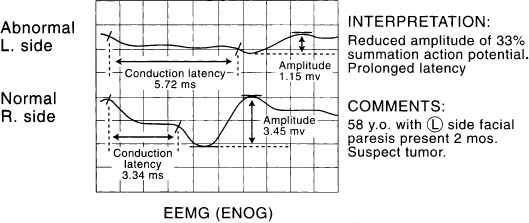
Figure 2-10. EEMG (ENOG) of patient in Figure 2-9. Reduced amplitude and prolonged conduction latency in a patient with a facial paresis that persists beyond 3 weeks without evidence of spontaneous recovery suggests the presence of a tumor. Appropriate imaging studies are indicated. In this case, imaging studies were normal. Based upon the pattern noted by EMG, surgical exploration of the facial nerve in the temporal bone and total parotidectomy was performed. An occult adenoid cystic carcinoma of the parotid gland was discovered.
Evoked electromyography has been quite helpful in predicting cases where the nerve has been affected either by a malignant or a benign tumor. Patients may present with minimal paresis (Fig. 2-9) and yet show marked reduction in the amplitude of the summation action potential and prolonged conduction latency, suggesting that the nerve has undergone degeneration and regeneration due to a slow ongoing process of invasion, replacement, and compression (Fig. 2-10). In patients with a parotid malignancy and facial paralysis or paresis prior to resection, the results of nerve grafting have been disappointing compared to those patients where the nerve was resected but was not involved with tumor. Clearly resection of benign tumors where the nerve was not involved (Fig. 2-11) and the nerve then repaired yielded ideal results much as one would expect if the nerve was transected iatrogenically or accidentally injuried and repaired immediately (Figs. 2-5 and 2-12).
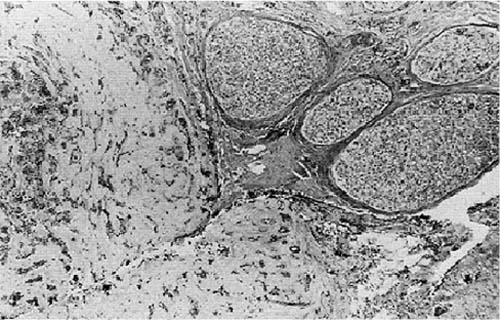
Figure 2-11. Section through tumor specimen taken from parotid of a patient (Fig. 2-12) with recurrent pleomorphic adenoma. The tumor is adherent to the facial nerve, but not invading. In such cases it is not possible to separate the tumor from the nerve and preserve facial nerve continuity.
Figure 2-12. A, Postresection of the facial nerve with the adherent pleomorphic adenoma and immediate nerve graft repair. B, C, Five years after surgery. Recovery with individual movement, some mass movement, and synkinesis. B, Smiling. C, Eyes closed.
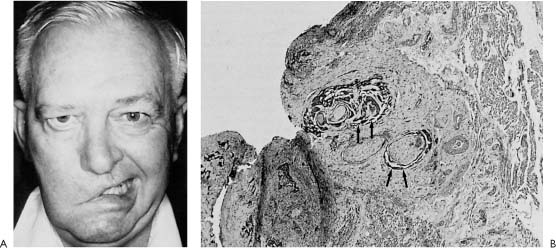
Figure 2-13. A, Patient presents with facial paralysis of 2 years duration diagnosed as “Bell’s palsy.” A history of radiation therapy for a recurrent basal cell carcinoma in the cheek skin on the same side a year prior to the onset of “Bell’s palsy” prompted an exploratory parotidectomy and facial nerve resection. B, Pathologic study of the specimen demonstrates basal cell carcinoma with perineural invasion (arrows).
In contrast, patients who preoperatively had a total paralysis with replacement or invasion of the facial nerve noted pathologically rarely showed any recovery following nerve repair (Fig. 2-13). The amount of facial recovery following resection and repair seem to depend on the amount of functioning fibers present at the time of surgery. Therefore, the surgeon can expect the amount of recovery following tumor resection and repair to vary from the most recovery in a patient who presents with normal function, to less recovery in a patient who presents with paresis, and little or no recovery in a patient who presents with total paralysis. This observation is quite helpful in planning reanimation procedures because one could depend solely on the nerve graft for recovery in the most favorable situation, while in the worst case, any recovery of facial function with a nerve graft would be unlikely. Further, in the poor prognostic situation, the patient’s overall prognosis for survival is questionable. In such a case, nerve repair that takes 2 years for full recovery may not be realistic, and it would be in the patient’s best interest to perform a procedure that accomplished immediate reanimation, such as a temporalis muscle transposition (Fig. 2-14). This principle should govern the reanimation surgeon’s decisions regarding whether to perform nerve grafts or some other technique. One should not expect to get a better facial recovery following resection and nerve repair than the amount of function the patient had prior to the surgery. In such cases, the distal end of the nerve to be repaired may show extensive collagenization because of the degeneration and regeneration process, and this is the major limiting factor in the amount of regeneration that is possible (Fig. 2-15).
Figure 2-14. Patient with a hard mass involving the left parotid and a total facial paralysis for 6 weeks. Incisional biopsy proved undifferentiated epidermoid carcinoma. A, Smiling. B, Closing the eye. C, Five weeks post-resection with upper eyelid spring insertion, lower eyelid tightening, cartilage implant, and temporalis transposition. Face at rest. D, Closing eyes. The animation techiques chosen achieved immediate facial rehabilitation in a patient with an advanced aggressive cancer. The prognosis for long-term survival was poor, and the likelihood of achieving useful facial nerve regeneration with nerve repair is unlikely in a patient with a preoperative total facial paralysis secondary to cancer invading the facial nerve.
The cause of injury, whether due to a knife, bullet, or shotgun wound, each have different considerations. A knife wound may sharply lacerate or separate the ends of the nerve allowing end-to-end repair (Fig. 2-16). An injury due to a bullet wound presents a different problem. Here the nerve may be disrupted, crushed, stretched, or cauterized by the heat generated as a high-velocity missile courses passed the vulnerable nerve (Fig. 2-17
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree