Fig. 6.1
Clinical morphology of MMP. (a) Inflammation of attached gingival; (b) Involvement of buccal mucosa; (c) Erosion of dorsal tongue; (d) Ocular inflammation and symblepharon; (e) Ocular ankyloblepharon
Ocular Lesions
Ocular involvement represents the second most common mucosal surface involved in MMP [1]. Ocular disease starts with inflammation and erosions on the lower bulbar conjunctiva, and blisters are not commonly observed. As inflammation continues, this process leads to symblepharon formation (Fig. 6.1d, e), and results in shortening of the fornix and in-turning of the eyelashes (entropion). Entropion defects cause irritation to the cornea, leading to corneal neovascularization, corneal opacification (scarring), and eventually blindness. Sometimes, fusion of the medial or lateral corners of the eye can occur, causing ankyloblepharon formation. Ocular involvement and disease staging have been discussed in a separate chapter on the treatment of ocular pemphigoid.
Genital Lesions
Although involvement of MMP on genital mucosae is not commonly observed, it can be very symptomatic in patients. The individual lesional morphology is very similar to that observed in the oral mucosa, and includes inflammation, blisters, erosions, and erythematous patches. However, if not treated at the onset of disease or treated insufficiently, the end result of genital mucosal lesions could involve significant stricturing of the genital orifice, and severe impairment of a patient’s normal functions of urination and sexual activities.
Other Mucosal Lesions
Rarely, MMP involves the esophageal, laryngeal, and anal mucosae. Dysphagia, hoarseness, and painful defecation are the symptoms and signs which alert physicians for possible involvement of these sites. To fully identify the lesions, most cases require endoscopic examination. The individual lesional morphology in these areas is not different from those observed in the oral mucosa. As is the case with the genital mucosa, sufficient treatment needs to be provided in a timely manner to prevent dysfunctional scarring from stricture formation.
Diagnostic Methods
Mucous membranes are common areas of involvement in multiple autoimmune blistering diseases that can mimic MMP. For example, the oral mucosa is affected in 70 % of pemphigus vulgaris (PV), and one study reported that more than 50 % of PV presents with the oral cavity as the primary site of involvement [4]. PV can also involve other similar mucosal surfaces as MMP, including the esophagus and genital areas [5, 6]. In addition, paraneoplastic pemphigus, which presents in patients with certain internal malignancies, characteristically manifests as a severe case of mucositis affecting multiple mucous membranes such as the oral, ocular, and genital regions [7]. Thus, utilization of accurate diagnostic methodologies is essential to distinguish MMP from other autoimmune blistering diseases. An algorithm for this purpose is depicted in Fig. 6.2. Starting from blistering diseases affecting mucosal surfaces, the histopathology of a biopsy specimen from lesional epithelial tissue will effectively divide patients into two distinct groups: the pemphigus group with intra-epithelial blisters, and the pemphigoid group with sub-epithelial blisters. Performing direct immunofluorescence (DIF) microscopy on peri-lesional epithelial tissue can allow for further examination of the pemphigoid group of patients. This would confirm the diagnosis of “pemphigoid” by the detection of immunoglobulin and/or complement component 3 at the epithelial basement membrane zone [1] (Fig. 6.3). Within this confirmed “pemphigoid” group, patients with predominantly mucosal disease will be classified as “MMP.” Based on other clinical and immunological features, patients with disease predominantly involving the skin surface will be categorized into other pemphigoid diseases, including linear IgA bullous dermatosis [8], epidermolysis bullosa acquisita [9], bullous pemphigoid [10], or two other rare pemphigoid diseases [11, 12]. Further sub-classification of MMP can be conducted through indirect immunofluorescence microscopy (IIF) on salt-split skin, and autoantigen identification by immunoblotting or enzyme-linked immunosorbent assay (ELISA) [13–19]. Despite the usefulness of MMP sub-classification, experts in the field consider that the general therapeutic strategy for all subgroups of MMP is essentially independent of this sub-classification result [1].
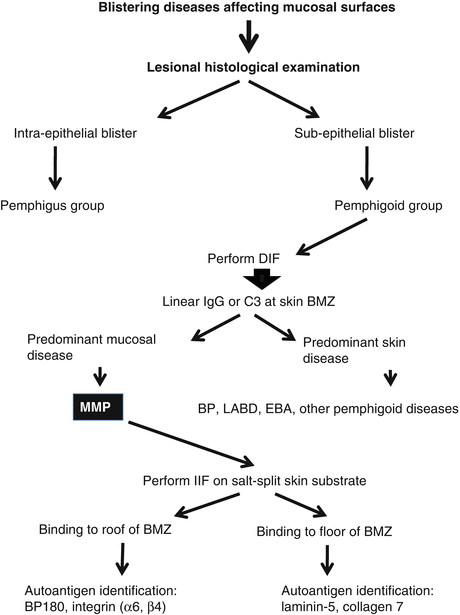
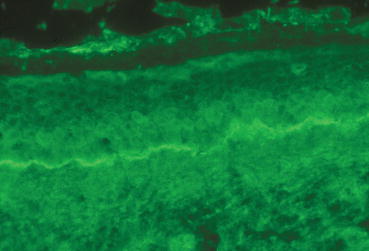

Fig. 6.2
Diagnostic methodology for MMP: an algorithm approach

Fig. 6.3
Immunopathology of MMP: direct immunofluorescence microscopic finding of linear IgG deposit in epithelial basement membrane
Treatment
The treatment of MMP can be challenging. This is due to the heterogeneous presentation of the disease along with a variable response to treatment. The current treatment options that are utilized are based on anecdotal case reports and case series. The therapeutic recommendations and algorithms have been made in consensus statements by panels of experts [1, 20]. A summary of the major studies, which were reviewed in a recent consensus statement, are presented in Tables 6.1 and 6.2, and covers the use of topical tacrolimus, laser therapy, and systemic treatments including minocycline, dapsone, azathioprine, mycophenolate mofetil, rituximab, and intravenous immunoglobulin (IVIg) [20–40]. The treatment of ocular pemphigoid was not included since this is reviewed in a separate chapter.
Table 6.1
Mucous membrane pemphigoid treatments
Treatment(s) | Patients | Clinical areas involved | Adjuvant treatment(s) | Results | Time to response | Follow-up | Previous treatments | Notes |
|---|---|---|---|---|---|---|---|---|
Tacrolimus [21] 0.1 % ointment QD | # 1 62 yo F | Genital | Pred 40 mg QD × 3 mos until CR, then stopped | CR | 3 mos | a | Pred 40 mg QD tapered to 10 mg + MMF 2 g QD AZA 150 mg QD | Resolution maintained 3+ mos on tacrolimus oint only |
Tacrolimus [22] 0.1 % ointment QD | # 2 67 yo M, 66 yo F | Oral | None | CR | 2–3 mos | 6–8 mos | Topical & systemic steroids, tetracycline, systemic retinoids, chloroquinea Specifics not listeda | Applied w dental swabs placed on areas for 15 min BID, mouth rinsed after removal of swabs Ointment Not swallowed Remission maintained during f/u |
Tacrolimus [23] 0.1 % ointment TID | # 1 70 yo F | Oral | None | MR | PR – 2 wks MR – 6 wks | 14 mos | Triamcinolone 0.1 % paste, intralesional steroida, Dex oral rinse (0.5 mg/5 cc) TID – QID | PR = 80 % lesion reduction MR = 95 % lesion reduction |
Tacrolimus [24] 0.03 % oral suspension 5 min rinse BID | # 1 84 yo M | Oral | None | CR | 2 mos | 4 mos | None | Tacrolimus tablets reconstituted in aqueous solution of tacrolimus 0.03 %, carboxymethylene 1 %, methyl parahydroxybenzoate 0.07 %, propylparahydroxybenzoate 0.03 %, water 98.9 % |
Minocycline [25] 100 mg BID initially | # 9 Mean 63.5 yo All F | Oral Aerodigestive | 5 Pts continued previous compounded clobetasol 0.05 % oint & 4 % hydroxyethyl cellulose gel BID | 3 × CR 4 × PR 2 × NR | 4–16 wks | Mean 4.5 yrs | Compounded clobetasol 0.05 % oint & 4 % hydroxyethyl cellulose gel BID | 5 Pts initially with compounded clobetasol 0.05 % oint 5 stopped due to adverse effects (vertigo > GI) High doses (200 mg/day) limited due to frequent adverse effects |
Mycophenolate sodium enteric-coated [26] Initially 360 mg BID, then 720 mg BID | # 2 63 yo M, 68 yo F | Mucosa Cutaneous | Mpred initially 1 mg/kg/d IV transitioned to oral 8 mg – 16 mg QD | 1 × CR 1 × PR | CR – 3 mos PR – 2 mos | 2–4 mos | Systemic corticosteroidsa CSAa | |
Cyclophosphamide [27] 2 mg/kg/day | # 13 6 M, 7 F Mean 69 yo (55–86 yo) | Oral Ocular Aerodigestive Genital | 10 Pts continued previous Dapa, Sulfasalazinea, topical agentsa | 7 × CR 2 × PR 4 × NR | PR to MR – 4–12 wks CR – 16–52 wks | ~12 mos | Dapa, Sulfasalazinea, topical agentsa | 3 Pts relapsed after CR (6–18 wks) Lymphopenia most common adverse effect Mean duration of CYCP administration 12 wks (range 2–52 wks) |
Multiple regimens [28] Azathioprine a Colchicine 1 mg – 1.5 mg QD (12 Pts) Cyclophosphamide 1 g QD (2 Pts) Dapsone 100 mg QD (10 Pts) | # 15 F:M = 2.8:1 Mean 63 yo (37–78 yo) | Oral Ocular Aerodigestive Genital | Pred 40 mg QD All patients except 1 | Colc – 5 × CR, 3 × PR Dap – 3 × CR AZA – 1 × PR CYCP – 1 × CR, 1 × PR | a | Mean 4 yrs (4 mos – 16 yrs) | a | Colc had an overall 67 % response rate Colc 1.5 mg dose did not offer any significant advantage, stopped due to diarrhea |
Combination therapy [29] Dapsone (initial 50 mg QD) Mycophenolate mofetil (initial 1–1.5 g QD) Prednisolone (initial 30–80 mg QD) | # 6 4 M, 2 F Mean 64.8 yo (43–86 yo) | Oral Ocular Aerodigestive Genital Cutaneous | Minocycline 100 mg then doxycycline a (1 Pt) Topical corticosteroidsa | CR | a | 18 mos | Majority received topical treatmenta + prednisolonea or Dapa | MMF (initial dose – 1 g/d × 5 Pts, 1.5 g/d × 1 Pt, adjusted at 18 mos to 0.5–1.5 g QD) Dap (initial dose – 50 mg/d, adjusted to 25–50 mg, 1 Pt stopped) Prednisolone (initial dose – 30 mg/d × 5 Pts, 80 mg/d × 1 Pt, tapered w remission at 18 mos to 5–10 mg QD to QOD) |
980 nm diode Laser [30] Low-level laser twice weekly – gallium-aluminum-arsenide diode laser (Laser settings under Notes) | # 3 1 M, 2 F Mean 79.4 yo (± 6.71) | Oral | a | CR | Mean 9.66 sessions (±4.72) | Mean 13.33 mos (±9.45 mos) | a | Laser Settings: diameter 0.6 cm, spot size 0.28 cmq, output power 300 mW, fluence 4 J/cmq @ 2 mm distance, scanning speed 1 cm/s 1 Pt had recurrence during f/u treated successfully with laser |
Table 6.2
Mucous membrane pemphigoid treatments
Treatment(s) | Patients | Clinical areas involved | Adjuvant treatment(s) | Results | Time to response | Follow-up | Previous treatments | Notes |
|---|---|---|---|---|---|---|---|---|
Etanercept [31] 25 mg SQ twice weekly | # 3 60 yo F, 47 yo F, 49 yo F | Oral Ocular | Pt 1 – AZA 100 mg BID + Dap 75 mg QD Pt 2 – IVIg a Pt 3 – nystatin swish + clobetasol oint | 1 × CR 2 × MR | CR – 1 mo MR – 1 mo
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



